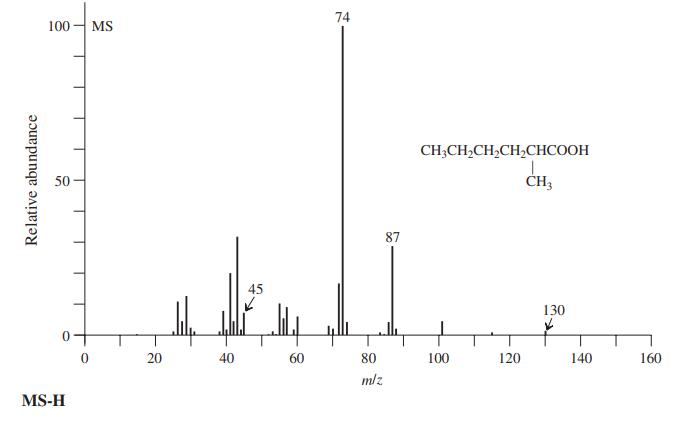
Interpret the labeled peaks in the mass spectrum of 2-methylhexanoic acid (MS-H). 74 100 MS CH;CH,CH,CH,CHCOOH 50
Question:
Interpret the labeled peaks in the mass spectrum of 2-methylhexanoic acid (MS-H).
Transcribed Image Text:
74 100 MS CH;CH,CH,CH,CHCOOH 50 CH3 87 45 130 20 40 60 80 100 120 140 160 mlz MS-H Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Indicate whether the following peaks in the mass spectrum of 1-heptanol are odd-electron or even-electron ions. (a) m/z = 83 (b) m/z = 70 (c) m/z = 56 (d) m/z = 41
-
a. Identify the fragments that would cause peaks in the mass spectrum of HOCH 2 COCH 3 with the following m/e values: i. m/e = 15 ii. m/e = 17 iii. m/e = 31 iv. m/e = 43 v. m/e = 57 vi. m/e = 59 b....
-
Three of the most intense peaks in the mass spectrum of 2-methyl-2-butanol appear at m/z 59, 70, and 73. Explain the origin of these peaks.
-
Doug Brackett wants to have enough mechanics on hand to take care of his customer requests, but he does not want to be paying mechanics to sit around doing nothing. Doug needs to know a reasonable...
-
Oligopoly, Increasing Returns, Tariffs and FD/. Recall from Chapter 3 that one motivation for setting up a branch plant in a foreign country is to avoid transport costs and tariffs, but this has to...
-
Describe, in pseudo-code, an algorithm for computing the number of descendents of each node of a binary tree. The algorithm should be based on the Euler tour traversal.
-
(Bond Theory: Balance Sheet Presentations, Interest Rate, Premium) On January 1, 2008, Branagh Company issued for $1,075,230 its 20-year, 13% bonds that have a maturity value of $1,000,000 and pay...
-
Arrow Distributing Corp. (See Table) likes to track inventory by using weeks of supply as well as by inventory turnover. (a) What is its weeks of supply? (b) What percent of Arrows assets are...
-
Calculate the euro-based return for an Italian investor who invested 10,000 into an American stock with stock price $50. One year after investment, the stock pays a $1 dividend and sells for $54....
-
The information listed below refers to the employees of Lemonica Company for the year ended December 31, 2016. The wages are separated into the quarters in which they were paid to the individual...
-
When methyl ketones are treated with a halogen in the presence of base, the three hydrogen atoms on the methyl carbon are replaced to give a CX 3 -substituted ketone. This product is not stable under...
-
Interpret the labeled peaks in the mass spectrum of 2-methylhexanoic acid (MS-H). 74 100 MS CH;CH,CH,CH,CHCOOH 50 CH3 87 45 130 20 40 60 80 100 120 140 160 mlz MS-H Relative abundance
-
What is sublimation?
-
Please help Calculating NPV and IRR Businesses use NPV and IRR to determine whether a project will add - value for shareholders. After watching the CFA Level I Corporate Finance video, answer the...
-
Assume that John wants to annuitize the annuity and is told that he can receive a straight life annuity for $600 a month for life. If the actuarial number of payments is 300, how much of the first...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 90% confident that the estimated percentage is in error...
-
Your homework for this week is to watch the first lecture on Financial Accounting and at the end of the outline there are several problems for you to do. The problems begin with parts A-D for you to...
-
Sheril Rose was a brilliant but penniless material scientist. She had designed a new type of solar panel she believed had great commercial potential. On January 15, she approached Felda Higgins, a...
-
Give amore efficient solution to the previous exercise that avoids the use of notify All. (It is tempting to observe that the buffer can never be both full and empty at the same time, and to assume...
-
Splitting hairs, if you shine a beam of colored light to a friend above in a high tower, will the color of light your friend receives be the same color you send? Explain.
-
Explain why the pKas of compounds near the middle of Table 4.2 are often listed with two figures to the right of the decimal place (that is, for NH4+ the pKa = 9.24), whereas those at the beginning...
-
For each pair of compounds, explain which is the stronger acid?
-
Explain why the compound on the left is a stronger acid than the compound on the right.
-
Becton Labs, Incorporated, produces various chemical compounds for industrial use. One compound, called Fludex, is prepared using Required: For direct materials: a . Compute the price and quantity...
-
Suppose you have $10,000 but you choose to borrow another $10,000 on margin allowing you to buy $20,000 in a stock with a price of $50 per share. If the stock price falls to $45 per share over the...
-
You sold a call option at strike 102 for a price of $4 and sold a put option at strike 98 for a price of $3, both options with the same maturity. In what range of stock prices at maturity will you...

Study smarter with the SolutionInn App



