Name each of the following compounds. (a) (b) (c) (d) (e) OH Cl, Br
Question:
Name each of the following compounds.
(a)
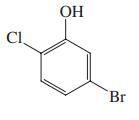
(b)
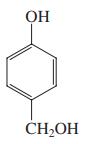
(c)
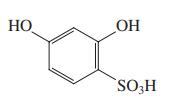
(d)
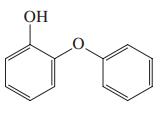
(e)
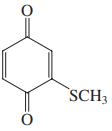
Transcribed Image Text:
OH ОН Cl, Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
a name is ortho chloro meta bromo phenole or 2 ch...View the full answer

Answered By

Sulochana kumawat
4 year experience . i am teacher . i am toutuber teaching on there / , chemistry is only my interested subject i choose this for
Teacher-Centered Methods of Instruction. Direct Instruction (Low Tech) Flipped Classrooms (High Tech) Kinesthetic Learning (Low Tech) Differentiated Instruction (Low Tech) Inquiry-based Learning (High Tech) Expeditionary Learning (High Tech) Personalized Learning (High Tech) Game-based Learning
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Name each of the following compounds using R,S and E,Z (Section 3.5) designations where necessary: a. b. c. d. e. f. g. h. CH2CH3 H- CH3 H2CH C-C C-C CI Br CH,CH CH-CH,CH,CH, - H3C CH2CH2l CH2CH...
-
Name each of the following compounds according to substitutive IUPAC nomenclature: (a) (CH3)2CHCH2CH2CH2Br (b) (CH3)2CHCH2CH2CH2OH (c) Cl3CCH2Br (d) (e) CF3CH2OH (f) (g) (h) (i) Cl2CHCHBr CI OH ,
-
Name each of the following compounds according to IUPAC? (a) (b) (c) CH3CH2CH2SO3H (d) CF3SO2CI. _CH2SH CH3 CH CH2CHSCH
-
The Whitewater LLP is equally owned by three partners and has the following balance sheet at the end of the current tax year: Partner Petula is an active (i.e., general) partner retiring from the...
-
Why did the Montreal Protocol succeed in limiting global emissions of chlorofluorocarbons (CFCs), whereas the world has found it difficult to limit the emissions of CO2? What differences between the...
-
Find the Taylor series with center z 0 and its radius of convergence. sin z, z 0 = /2
-
8. Use the method of Example 7.36 to estimate 1f'(x)l.
-
After some further analysis, you discover that the commission field in the Policies table is updated yearly to reflect changes in the annual commission paid to agents on existing policies. Would...
-
if bondholder holds one premium and one discount bond , should the bondholder exercise the conversion for either of the bonds?
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Starting with benzene, propose syntheses of each of the following phenol derivatives. (a) (b) (c) - CI Ci The herbicide 2,4-D
-
Give the expected product(s) of each of the following reaction sequences. (a) (b) (c) (d) (e) (e) 1.2 CH, Br, NaOH 2.
-
What role do intelligent agents play in the operation of a decision-support system?
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Solve the following linear system by Gaussian elimination with back-substitution without introducing fractions in your row-reduction. If there is no solution, explain why. -3x+8y + 82 = -8 -2x+ y -...
-
Introduction Some predictions are a slam dunk. Retail will continue to be driven by technology. Science fiction is coming to life in the form of robotics and virtual reality. And the Internet will...
-
Oswego Clay Pipe Company provides services of $ 5 0 , 0 0 0 to Southeast Water District # 4 5 on April 1 2 of the current year with terms 1 / 1 5 , n / 6 0 . What would Oswego record on April 1 2 ?...
-
Assume the following excerpts from a company's balance sheet: Property, plant, and equipment Beginning Balance $3,500,000 Ending Balance $3,700,000 $1,100,000 $800,000 Long-term investments During...
-
Consider the following program, saved into a file named Example.java: What would happen if each of the following changes were made to the Example program? For example, would there be no effect, a...
-
The National Collegiate Athletic Association (NCAA) and the National Federation of State High School Associations (NFHS) set a new standard for non-wood baseball bats. Their goal was to ensure that...
-
Predict the multiplicity of the absorption for Hm if Jam = Jmx. Explain. , , . -C-C-C-
-
Construct a tree diagram for the absorption of Hm assume that Jam . . - -C-
-
Predict the multiplicities of the absorptions for the hydrogen's of these groups, assume that hydrogen's labeled a are different from those labeled x but that all of those labeled a are identical and...
-
Full-line selling, or suggestion selling, should be viewed as a form of customer service. O True False
-
Fielding Wilderness Outfitters has projected its sales for the first six months of 2008 to be as follows: Jan- $50,000, April $180.000. Feb-$60,000, May $240,000. Mar-$100,000, June $240,000 Cost of...
-
As William is preparing the end of year financial statements, he notices that the numbers required for his personal bonus have not been met. He begins to review the estimates that he has made in...

Study smarter with the SolutionInn App


