Predict the 13 C NMR spectra of the compounds in Problem 36, with and without proton decoupling.
Question:
Predict the 13C NMR spectra of the compounds in Problem 36, with and without proton decoupling.
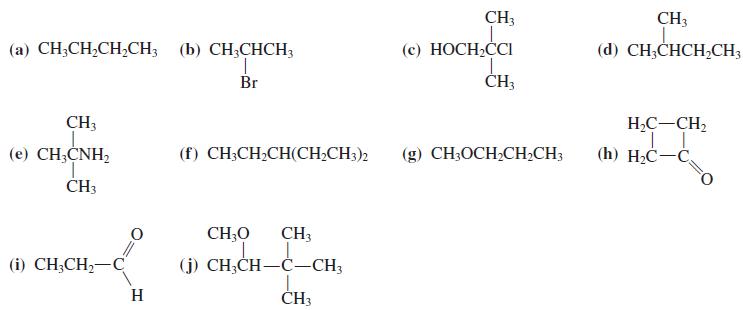
Transcribed Image Text:
CH3 CH3 (с) НОСН,СCI CH,CI (a) CH;CH,CH,CH; (b) CH;CHCH3 (d) CH;CHCH,CH3 Br ČH3 CH3 H,C-CH, (е) СН,CNH, (f) CH;CH;CH(CH;CH3)2 (g) CH3OCH;CHCH3 (h) H2C-C ČH3 CH;O CH3 (i) CH,CH— С (j) CH;CH-C-CH3 H ČH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
SNo with coupling without coupling a C1q2C C2t2C al...View the full answer

Answered By

Bharat Singh Patel
I like teaching since my matriculation. That time i was teaching 6-8 class students with all subjects. I was very interested in teaching. Then after i have done my 10+2. I joined a coaching class. Where i was teaching mathematics and chemistry of 10th class student. After that i moved to Ewing Christian College, Allahabad for Graduation. then i taught chemistry only. then I clear IIT JAM to got admission in IIT Jodhpur for M.Sc. During M.Sc I have cleared CSIR-JRF With AIR 54 and GATE with AIR 114. In college time I always cleared doubts of classmates and juniors.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Predict the number of carbon resonance lines you would expect in the 13C NMR spectra of the following compounds: (a) Methylcyclopentane (b) 1-Methylcyclohexene (c) 1, 2-Dimethylbenzene (d)...
-
Draw the expected broadband-decoupled 13C NMR spectra of the following compounds. Use Figure 13-41 (page 603) to estimate the chemical shifts. (a) (b) (c) (d) C CH H,C CH C-H C-C
-
Repeat Problem 13-25, sketching the off-resonance-decoupled 13C spectra of the compounds. Problem 13-25 Draw the expected broadband-decoupled 13C NMR spectra of the following compounds. Use Figure...
-
Since perpetuity payments continue forever, how can a present value be computed? Why isnt the present value infinite?
-
Following are four short case study scenarios that represent risk response category choices. Match each scenario with one of the four possible risk responses (accept, avoid, transfer, mitigate) and...
-
Why does byte code make Java a portable language?
-
Work-life balance is just another fad. In a few years time it will be superseded by another issue. To what extent do you agree with this statement and why? LO1
-
For each of the following brief scenarios, assume that you are reporting on a clients financial statements. Reply as to the type(s) of opinion possible for the scenario. In addition: Unless stated...
-
The following is the Bravo Unlimited adjusted Trial Balance. Bravo Unlimited Adjusted Trial Balance 31-Dec-17 Account Title Debit Credit Cash $88,450 Accounts Receivable 331,860 Supplies 11,255...
-
Electromagnetic technologies offer effective nondestructive sensing techniques for determining characteristics of pavement. The propagation of electromagnetic waves through the material depends on...
-
Can the three isomeric pentanes be distinguished unambiguously from their broad-band proton-decoupled 13 C NMR spectra alone? Can the five isomeric hexanes be distinguished in this way?
-
Rework Problem 37 as it pertains to 13 C NMR spectroscopy. Rework Problem 37 For each compound in each of the following groups of isomers, indicate the number of signals in the 1 H NMR spectrum, the...
-
The author surveyed some of the students in one of his statistics classes about the distances (in miles) they commute to college. Their responses are described by the histogram shown in Fig. 85. a....
-
Which of the following is not included in the cash flow statement? a. Cash from short-term investments b. Cash from operations c. Cash from the balance sheet d. Cash from capital financing Which of...
-
Case Study Chapter 13B Pharm - Saved Case Study Chapter 13 Central Nervous System Stimulants and Related Drugs Nancy has been unsuccessful in preventing migraine headaches and has been prescribed a...
-
Toro Corp. reports the following two years of balance sheets and some additional information. 2019 2018 Cash S 92,915 $ 31,355 Accounts receivable 94,000 80,850 Inventory 179,000 157,600 Prepaid...
-
The Westchester Chamber of Commerce periodically sponsors public service seminars and programs. Currently, promotional plans are under way for this year's program. Advertising alternatives include...
-
Mastery Problem: Differential Analysis and Product Pricing WoolCorp WoolCorp buys sheep's wool from farmers. The company began operations in January of this year, and is making decisions on product...
-
Write a method called printRange that accepts two integers as arguments and prints the sequence of numbers between the two arguments, separated by spaces. Print an increasing sequence if the first...
-
According during to the IRS, individuals filing federal income tax returns prior to March 31 received an average refund of $1,088 in 2018. Consider the population of "last-minute" filers who mail...
-
The mass density of water vapour at 327.6 atm and 776.4 K is 133.2 kg m-3. Given that for water Tc = 647.4 K, Pc = 218.3 atm, a = 5.464 dm6 atm mol-2, b= 0.03049 dm3 mol-1, and M= 18.02 g mol-1,...
-
Estimate the coefficients a and b in the Dieterici equation of state from the critical constants of xenon. Calculate the pressure exerted by 1.0 mol Xe when it is confined to 1.0 dm3 at 25C.
-
Express the van der Waals equation of state as a virial expansion in powers of 1/Vm and obtain expressions for Band C in terms of the parameters a and b. The expansion you will need is (1- xtI = 1 +...
-
a. Determine the equal annual net cash flows from operating the bulldozer. Use a minus sign to indicate cash outflows
-
Rose (a single taxpayer) is the owner of Americana, LLC. The LLC (a sole proprietorship) reports QBI of $900,000 and is not a specified services business. Americana paid total W-2 wages of $300,000,...
-
On May 31, the Cash account of Teasel had a normal balance of $7,000. During May, the account was debited for a total of $14,200 and credited for a total of $13,500. What was the balance in the Cash...

Study smarter with the SolutionInn App


