Propose a mechanism for the sulfonation of benzene using chlorosulfuric acid, ClSO3H (in the margin). Cl-S-OH SO,H
Question:
Propose a mechanism for the sulfonation of benzene using chlorosulfuric acid, ClSO3H (in the margin).
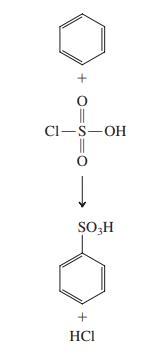
Transcribed Image Text:
Cl-S-OH SO,H + HCI + O=S
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
Macho footne sulphmation of ...View the full answer

Answered By

Ketankumar amlani
I completed my bachelor degree in 2012 with 72.57%
i completed my master degree in 2014 with 67.50%
i completed my bachelor of education in 2019 with 87.50%
I qualified GATE (graduate aptitude test in engineering) examination in 2020
I qualified GSET (Gujarat state eligibility test) examination with highest marks in Gujarat.
I am doing personal coaching from 2014 to till date.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Write out the steps in the mechanism for the sulfonation of benzene.
-
Propose a mechanism for the acid-catalyzed bromination of pentan-3-one.
-
Propose a mechanism for the sulfonation of pyridine, pointing out why sulfonation occurs at the 3-position.
-
Find a harmonic function (x, y) in the right-half plane x > -1 such that on the boundary (-1, y) = 0, y
-
Explain what is meant by the environment surrounding a project. How is a project affected by events in the environment?
-
Which of the following information security roles is accountable for the day-to-day operation of the information security program? a. Security Analyst b. CISO c. CSO d. Security Manager
-
In much discrimination legislation an important distinction is made between direct and indirect discrimination. The former relates to a situation in which someone is discriminated against because of...
-
Sashas Foods produces frozen meals, which it sells for $7 each. The company uses the FIFO inventory costing method, and it computes a new monthly fixed manufacturing overhead rate based on the actual...
-
20. Discuss the effectiveness of SFAS No. 13 in addressing the lease capitalization problem.
-
Assume an accounting professor at the University of Texas devotes 60% of her time to teaching, 30% of her time to research and writing, and 10% of her time to service activities such as committee...
-
Hexadeuteriobenzene, C 6 D 6 , is a very useful solvent for 1 H NMR spectroscopy because it dissolves a wide variety of organic compounds and, being aromatic, is very stable. Suggest a method for the...
-
Benzene reacts with sulfur dichloride, SCl 2 , in the presence of AlCl 3 to give diphenyl sulfi de, C 6 H 5 S C 6 H 5 . Propose a mechanism for this process.
-
Suppose you know that the distribution of sample proportions of nonresidents in samples of 200 students is normal with a mean of 0.34 and a standard deviation of 0.03. Suppose you select a random...
-
21. Define subjective brightness and brightness adaptation 22. Define weber ratio 23. What is meant by machband effect? Machband effect means the intensity of the stripes is constant. Therefore it...
-
26. Define sampling and quantization 27. Find the number of bits required to store a 256 X 256 image with 32 gray levels 28. Write the expression to find the number of bits to store a digital image?...
-
31. What is meant by path? 32. Give the formula for calculating D4 and D8 distance. 33. What is geometric transformation? 34. What is image translation and scaling? 35. Define the term Luminance
-
1. Explain Brightness adaptation and Discrimination 2.Explain sampling and quantization:
-
3. Explain about Mach band effect? 4. Explain color image fundamentals. 5. Explain CMY model.
-
Arrow Manufacturing Co. expects to make 50,000 chairs during the Year 1 accounting period. The company made 3,000 chairs in January. Materials and labor costs for January were $36,000 and $48,000,...
-
The packaging division of a company having considered several alternative package designs for the company's new product has finally brought down their choices to two designs of which only one has to...
-
The data from a sedimentation equilibrium experiment performed at 293 K on a macromolecular solute in aqueous solution show that a graph of in c against (r/cm) 2 is a straight line with a slope...
-
Calculate the radial acceleration (as so many g) in a cell placed at 5.50 cm from the centre of rotation in an ultracentrifuge operating at 1.32 kHz.
-
A polymer chain consists of 1200 segments, each 1.125 nm long. If the chain were ideally flexible, what would be the Lm S. separation of the ends of the chain?
-
Match each of the following transactions with the applicable internal control principle that is being violated
-
Vaughn Company sells two types of pumps. One is large and is for commercial use. The other is smaller and is used in residential swimming pools. The following inventory data is available for the...
-
To fund your dream around-the-world vacation, you plan to save $1,300 per year for the next 14 years starting one year from now. If you can earn an interest rate of 5.83 percent, how much will you...

Study smarter with the SolutionInn App


