Propose a reasonable structure for calcium carbide, CaC 2 , on the basis of its chemical reactivity
Question:
Propose a reasonable structure for calcium carbide, CaC2, on the basis of its chemical reactivity (Section 13-10). What might be a more systematic name for it?
Section 13-10
Ethyne chemistry underwent important commercial development in the 1930s and 1940s in the laboratories of Badische Anilin and Sodafabriken (BASF) in Ludwigshafen, Germany. Ethyne under pressure was brought into reaction with carbon monoxide, carbonyl compounds, alcohols, and acids in the presence of catalysts to give a multitude of valuable raw materials to be used in further transformations. For example, nickel carbonyl catalyzes the addition of carbon monoxide and water to ethyne to give propenoic (acrylic) acid. Similar exposure to alcohols or amines instead of water results in the corresponding acid derivatives. All of these products are valuable monomers (see Section 12-15).
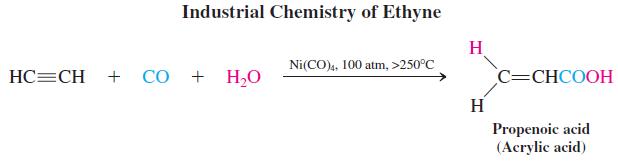
Polymerization of propenoic (acrylic) acid and its derivatives produces materials of considerable utility. The polymeric esters (polyacrylates) are tough, resilient, and flexible polymers that have replaced natural rubber (see Section 14-10) in many applications. Poly(ethyl acrylate) is used for O-rings, valve seals, and related purposes in automobiles. Other polyacrylates are found in biomedical and dental appliances, such as dentures.
The addition of formaldehyde to ethyne is achieved with high efficiency by using copper acetylide as a catalyst.
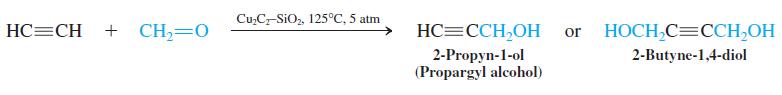
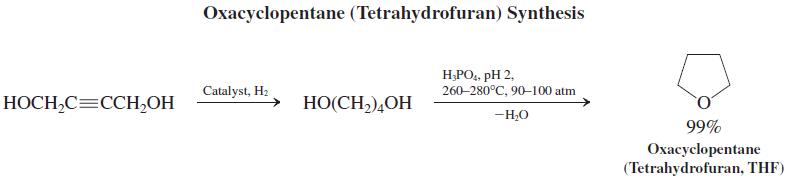
The resulting alcohols are useful synthetic intermediates. For example, 2-butyne-1,4-diol is a precursor for the production of oxacyclopentane (tetrahydrofuran, one of the solvents most frequently employed for Grignard and organolithium reagents) by hydrogenation, followed by acid-catalyzed dehydration.
Several technical processes have been developed in which reagents δ+A – Bδ+ in the presence of a catalyst add to the triple bond. For example, the catalyzed addition of hydrogen chloride gives chloroethene (vinyl chloride), and addition of hydrogen cyanide produces propenenitrile (acrylonitrile).
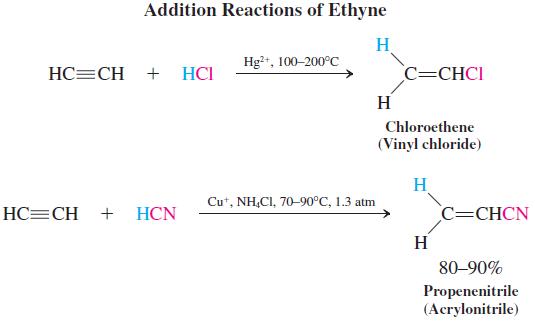
In 2007, the world produced 2.5 million tons of acrylic fibers, polymers containing at least 85% propenenitrile (acrylonitrile). Their applications include clothing (Orlon), carpets, and insulation. Copolymers of acrylonitrile and 10 – 15% vinyl chloride have fire-retardant properties and are used in children’s sleepwear.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





