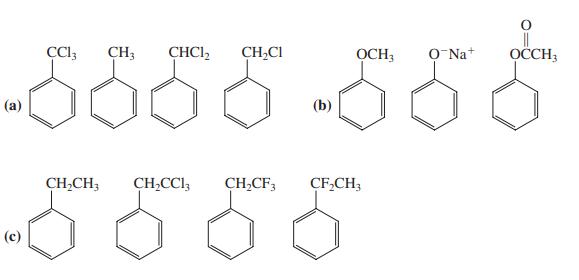
Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution.
Question:
Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain your rankings.
Transcribed Image Text:
|| OCCH3 CH3 CHCI, CH,CI OCH; O Na+ (а) (b) CH,CH; CH,CCl, CH,CF, CF,CH; (с)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a Let first compound 1 second compound 2 third compound 3 fourth compound 4 We know that alpha hydro...View the full answer

Answered By

Ankan Nath
I love to teach. All throughout school I have helped fellow students and juniors with their doubts. I believe that teaching concepts is beneficial for both the students, as they get to learn, and teachers, as they get to clear their concepts and gaps in understanding. I am an ardent lover of science and the scientific method, which I try to integrate into my teaching method. I am good at teaching both concepts and important problem solving skills. Mathematical understanding of concepts is something I stress upon. I have cleared multiple national level entrance examinations with good ranks. Teaching is my passion, and I hope to pursue a career in academics. I hope to help students on this amazing platform.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
List the compounds in each of the following groups in order of decreasing acidity: a. b. c. CH2-CH2 CH3CH3 CH3CH HC=CH 0 O CH CCH,CCH CH CCH2COCH
-
Rank the compounds in each group in order of decreasing acidity. (a) CH3CHCICH20H, CH3CHBrCH2OH, BrCH2CH2CH2OH (b) CH3CH2CH2OH, CCI3CH2OH, (CH3)2CC1CH2OH (c) (CH3)2CHOH, (CF3)2CHOH, (CC13)2CHOH
-
Rank the compounds in order of decreasing λ max: CH CH CH CH2
-
Develop a two-period weighted moving average forecast for periods 12 through 15. Use weights of 0.7 and 0.3, with the most recent observation weighted higher. PERIOD DEMAND 10............ 248...
-
This case concerns statements made by a prosecutor in his closing argument to the jury. In a capital murder case, the prosecutor made comments about the fact that the state of mind of the defendant...
-
This String class method performs the same operation as the + operator when used on strings. a. Add b. Join c. Concat d. Plus
-
1 What do they tell you about working there? What seem to be the most prominent features? Visit the websites of companies that interest you, perhaps as possible places to work. www.childbase.co.uk...
-
Nate Young is the dean of a business school. The university is under strong financial pressures, and the university president has asked all the deans to cut costs. Nate is wondering how he should...
-
Your corporation is considering whether to undertake a project or not. The project requires the purchase of a machine costing $500,000. The machine has a useful life of five years and a CCA rate of...
-
1. East Coast Yachts uses a small percentage of preferred stock as a source of financing. In calculating the ratios for the company, should preferred stock be included as part of the companys total...
-
One of the compounds shown here contains carbon carbon bonds that are 1.39 long. Which one? CH3 () (b) () -CH3 (d) (e) H;CC=CCH; CH3
-
Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain your rankings. || OCCH3 CH3 CHCI, CH,CI OCH; O Na+ () (b) CH,CH;...
-
In a discussion of accidental deaths involving roadside hazards, the web site highwaysafety.com included a pie chart like the one shown: a. Do you think this is an effective use of a pie chart? Why...
-
Officials of Gwinnett County, one of the fastest growing counties in the country, are looking for ways to expand their sewer system. They are considering two alternative sewer designs. All annual...
-
On August 1, 20Y7, Rafael Masey established Planet Realty, which completed the following transactions during the month: Rafael Masey transferred cash from a personal bank account to an account to be...
-
The following information summarizes the activities in the Mixing Department for the month of March. Beginning inventory 1 , 0 0 0 units, 8 0 % complete Started and completed 2 4 , 5 0 0 units Ending...
-
What is your recommendation for the maximum size of coarse aggregate for the following situation? A continuously reinforced concrete pavement cross section contains a layer of No. 6 reinforced bars...
-
On January 1, 2024, Winn Heat Transfer leased office space under a three-year operating lease agreement. The arrangement specified three annual lease payments of $72,000 each, beginning December 31,...
-
Selected financial information for Edwards Company for Year 4 follows. Sales ..................................... $800,000 Cost of goods sold ................ 500,000 Merchandise inventory Beginning...
-
Pearson Education, a publisher of college textbooks, would like to know if students prefer traditional textbooks or digital textbooks. A random sample of students was asked their preference and the...
-
The pure rotational microwave spectrum of HCI has absorption lines at the following wave numbers (in cm-1): 21.19, 42.37, 63.56, 84.75, 105.93, 127.12148.31169.49,190.68,211.87,233.06,254.24, 275.43,...
-
For H2 at very low temperatures, only translational motion contributes to the heat capacity. At temperatures above eR = luBlk, the rotational contribution to the heat capacity becomes significant. At...
-
Show that the mean interaction energy of N atoms of diameter d interacting with a potential energy of the form C61R6 is given by U=-2NzC6/3Vd3, where V is the volume in which the molecules are...
-
Save 6 Halles Betty Farm establishes a $200 petty cash fund on September 4 to pay for minor cash expenditures. The fund is replenished at the end of each month At the end of September, the fund...
-
MERCHANDISING ACOUNTING Joe Blink opened Blink Corporation. It has issued 20,000 shares of $5 par value common stock. It authorized 900,000 share. The corporation is a merchandising business. Blink...
-
Hi! I need to prepare a tax internal research memo to file. Facts: Don has a very painful terminal disease and has learned that marijuana may mitigate his pain. Don lives in a state in which it is...

Study smarter with the SolutionInn App


