Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution.
Question:
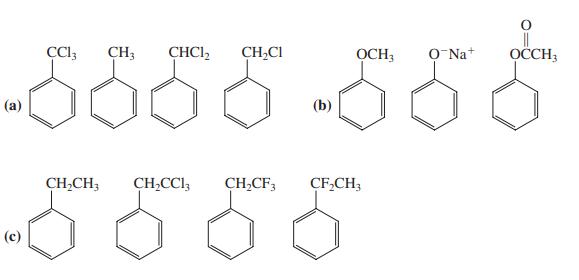
Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain your rankings.
Transcribed Image Text:
|| OCCH3 CH3 CHCI, CH,CI OCH; O Na+ (а) (b) CH,CH; CH,CCl, CH,CF, CF,CH; (с)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Electrophilic reactions are those in which electrophile attacks t...View the full answer

Answered By

Vivek Kumar
Hi. I am Postgraduate in Chemistry (2012) with First class degree.
Expert in time bound exam assignment and quizzes. Did for many students
Seasoned online chemistry Tutor with 4 years of experience in tutoring across the globe.
Students loves my lessons and i get lots of appreciations and complements. I teach every part of chemistry ranging from Physical chemistry to Medicinal chemistry.
If you ask me speciality, i would say Organic chemistry, Spectroscopy (MS, NMR IR and UV etc) and Physical chemistry.
I am also tutoring maths.
I believe teaching in an art. Not every student is same and so the subject. Therefore it is the responsibility of Tutor to use that art to help students and make the subject interesting.
Thank You. Keep Smiling and keep learning
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
List the compounds in each of the following groups in order of decreasing acidity: a. b. c. CH2-CH2 CH3CH3 CH3CH HC=CH 0 O CH CCH,CCH CH CCH2COCH
-
Rank the compounds in each group in order of decreasing acidity. (a) CH3CHCICH20H, CH3CHBrCH2OH, BrCH2CH2CH2OH (b) CH3CH2CH2OH, CCI3CH2OH, (CH3)2CC1CH2OH (c) (CH3)2CHOH, (CF3)2CHOH, (CC13)2CHOH
-
Rank the compounds in order of decreasing λ max: CH CH CH CH2
-
i) Starbucks launched its prepaid (debit) Starbucks Card in November 2001. The card, which holds between $5 and $500 can be used at any Starbucks location. Suppose Starbucks management wants to study...
-
This case concerns statements and evidentiary rulings made by a judge during the course of a criminal trial. The judge interrupted defense counsel during the opening statements telling him to "get to...
-
Many companies use telephone numbers like 555-GET-FOOD so the number is easier for their customers to remember. On a standard telephone, the alphabetic letters are mapped to numbers in the following...
-
1 Analyse these accounts using Herzbergs theory which of his motivating factors do staff refer to?
-
1. Assume you have AUD100,000 that you must invest in stocks. Construct a portfolio of 5 stocks from the data provided. Design the portfolio in any which way you deem fit. Justify your portfolio...
-
Questions: Report: Apple Inc. - Analysis of Fiscal 2017 Annual Report Apple Inc. is an American multinational technology company that designs, develops, and sells consumer electronics, computer...
-
The co-op student working at Morneau Equipment Inc. prepared their adjusted trial balance in alphabetical order. All accounts have their normal balances. Required 1. Prepare the appropriate closing...
-
Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic substitution. Explain your rankings. || OCCH3 CH3 CHCI, CH,CI OCH; O Na+ () (b) CH,CH;...
-
The rate of nitration of (chloromethyl)benzene, shown below is 0.71 relative to the rate of nitration of benzene (=1). The (chloromethyl)nitrobenzene product mixture that results contains 32% ortho,...
-
Find two sets of parametric equations for the rectangular equation. y = x 3 + 3
-
A closed square pyramid tank (base width: 6.0 m; height 3.0 m), sitting on its square base, has a 1.0 m depth of water. Suppose this tank is inverted (turned upside down) and is made to stand on its...
-
P.4.3 Apply a Taylor series expansion to a mixed backward formula for the first derivative: (Ux)i = 1 Ax (aui-2+ bui-1 + cu + dui+1) Derive the family of second order accurate formulas and the...
-
Show how you would go about balancing the following equations: Cu + HNO3 Cu(NO3)2 + NO + H2O HIO3 + Fel2 + HCI FeCl3 + ICI + H2O 2.Conservation of mass A student places 0.58 g of iron and 1.600 g...
-
Sales MOSS COMPANY Income Statement For Year Ended December 31, 2021 Cost of goods sold Gross profit Operating expenses (excluding depreciation) Depreciation expense Income before taxes Income taxes...
-
Prior to the Covid-19 epidemic, Master's and Ph.D. programs in psychology required applying students to submit their scores on the standardized graduate admission exam (GRE). For the past three...
-
In July 2015, H. J. Heinz Company and Kraft Foods Group completed their merger. The new company, named The Kraft Heinz Company (KHC), became the fifth largest food and beverage company in the world....
-
Quality Chicken grows and processes chickens. Each chicken is disassembled into five main parts. Information pertaining to production in July 2012 is: Joint cost of production in July 2012 was $50. A...
-
Find the drift speed of a particle of radius 15.5m and density 1250 kg m-1 which is settling from suspension in water (density 1000 kg m-1) under the influence of gravity alone. The viscosity of...
-
Calculate (a) The thermal wavelength, (b) The translational partition function of an Ar atom in a cubic box of side 1.00 cm at (i) 300 K and (ii) 3000 K.
-
Use the kinetic theory to justify the following observations: (a) The rate of a reaction in the gas phase depends on the energy with which two molecules collide, which in turn depends on their...
-
Which of the following statements regarding the Net Present Value (NPV) is incorrect ? A. If a projects NPV is positive, its IRR is also positive. B. NPV is the PV sum of all (positive and negative)...
-
In 2022, Mike incurred $2,500 in unreimbursed employee business expenses (none of the expenses are impairment-related). Her adjusted gross income was 50,000, and she had no other miscellaneous...
-
A man borrows $10,000 to be paid back in 30 years with level end of year payments at an annual loan rate of i. The sum of principal payments in years 5 and 10 is equal to the principal repaid in year...

Study smarter with the SolutionInn App


