Split your team in two, each group to analyze one of the following reaction sequences by a
Question:
Split your team in two, each group to analyze one of the following reaction sequences by a mechanism (13C 5 carbon-13 isotope).
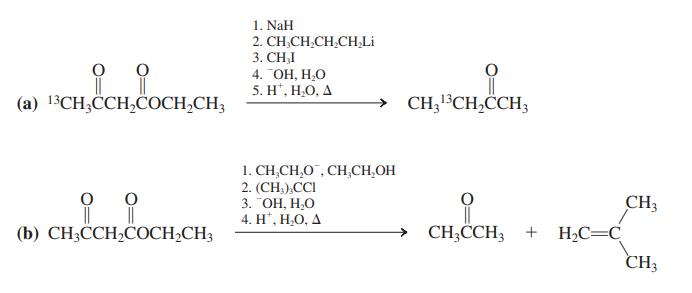
Reconvene and discuss your results. Specifi cally, address the position of the 13C label in the product of (a) and the failure to obtain alkylation in (b).
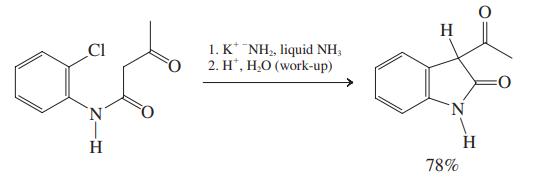
As a complete team, also discuss the mechanism of the following transformation.
Transcribed Image Text:
1. NaH 2. CH,CH,CH,CH,Li 3. СHI 4. ОН, Н.О 5. H", Н.О, Д (а) 13CH,ССH,CОСH-CH CH;13CH,CCH3 1. CH,CH,O , CH,CH,OH 2. (CH,),CCI 3. "ОН, Н.О 4. H", Н.О, А CH3 → CH;CCH3 + H2C=C CH3 (b) СH,ССH,CОCH-CH;
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
The reaction sequence involves the conversion of CH3CH2CH2CH2Li 2 into CH3CH2CH2CH2OH 4 The steps in...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Your task is to prepare isopropyl methyl ether by one of the following reactions. Which reaction would give the better yield? Explain your answer. (1) Isopropyl methyl ether Or (2) Isopropyl methyl...
-
One way to prepare esters is by the reaction of an alcohol with an acid anhydride (as in this experiment). However, they also may be prepared by the reaction between an alcohol and a carboxylic acid...
-
This problem introduces two literature syntheses of indole derivatives, and you are asked to come up with plausible mechanisms for them. Divide your team in two, each group concentrating on one of...
-
What effect does a weakening in U.S dollar have on Net Income for subsidiaries of U.S companies?
-
In your answer to this question, use a diagram and start from a no-trade point like S0 with a no-trade price ratio of 2 W/C. Now trade is opened and the country can trade whatever it wants at an...
-
Model and solve by the Laplace transform: Find the current i(t) in the RC-circuit in Fig. 150, where R = 10 , C = 0.1 F, v(t) = 10t V if 0 4, and the initial charge on the capacitor is 0.
-
2.2 Limit Theorems 1. (c) You may use Exercise 4. 2. (a) -3. (b) 1/5. (c) ,j2 (see Exercise 4). (d) O.
-
Identify specific fraud risk factors present during PwCs audits of the Lipper hedge funds. Explain how PwC should have responded to the fraud risk factors that you identified.
-
Suppose you sell a put option on ABC Inc. for $3.25 that expires in three months with a strike price of $40.00. Three months later, at expiration, ABC is trading at $42.00 per share. Your net...
-
What attachment pattern did Timmy display when Vanessa picked him up from child care, and what factors probably contributed to it?
-
Propose a synthesis of ketone C, which was central in attempts to synthesize several antitumor agents. Start with aldehyde A, lactone B, and anything else you need. H,C H2C A B C
-
Two of the following four compounds are more acidic than CH 3 OH (i.e., two of these have K a greater than methanol). Which ones? (a) A and B; (b) B and C; (c) C and D; (d) D and A; (e) D and B....
-
Methane gas (CH4) at 25C is burned with the stoichiometric amount of air at 25C during an adiabatic steady-flow combustion process at 1 atm. Assuming the product gases consist of CO2, H2O, CO, N2,...
-
Q9 (5 points) According to Dr. Henry Mintzberg, a noted management scholar from McGill University in Montreal, PQ, "business organizations perform only two activities of consequence." What are these...
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28W / (m ^ 2) ."C. The thermal conductivity of the solid...
-
Which of the following best demonstrates the Six Cs of Communication, "you" approach, and positive emphasis? Question 1 4 options: It will be February 1 0 before you will receive your materials. It...
-
please answer all the questionss.,.within 30 minutes. make sure the explanation and reasons are explained in very detailed manner as in why the chosen option is right and why other options are wrong....
-
1) A net force of 20 N is applied to the right on an object. If the acceleration of the object is 2.5 m/sec, a) What is the mass of the object? (8 kg) b) What is the weight of the object? (78.4 N) c)...
-
Suppose that you have a variable called line that will take on the values 1, 2, 3, 4, and so on, and a class constant named SIZE that takes one of two values. You are going to formulate expressions...
-
How can NAFTA be beneficial to suppliers of Walmart?
-
Fluoromethane (CH3F, = 1.81 D) has a smaller dipole moment than chloromethane (CH3C1, = 1.87 D) even though fluorine is more electronegative then chlorine. Explain
-
Methanethiol, CH2SH, has a substantial dipole moment ( = 1.52) even though carbon and sulfur have identical electro negativities. Explain.
-
Calculate the formal charges on the atoms shown inred. (a) (CH3)2OBF3 (b) H2C-NEN: (c) H2C=N=N: (d) :=-: (e) H (f) - CH
-
For anOld Country Links, Incorporated, produces sausages in three production departments Mixing , Casing and Curing, and Packaging. In the Mixing Department, meats are prepared, ground and mixed with...
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2

Study smarter with the SolutionInn App


