Calculate the formal charges on the atoms shown inred. (a) (CH3)2OBF3 (b) H2C-NEN: (c) H2C=N=N: (d) :=-:
Question:
Calculate the formal charges on the atoms shown inred.
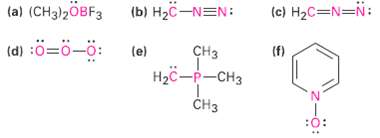
Transcribed Image Text:
(a) (CH3)2OBF3 (b) H2C-NEN: (c) H2C=N=N: (d) :ö=ö-ö: (e) сHз (f) Нас —р-снз CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
To save space molecules are shown as linebond structures with lone pairs rather than as electrondo...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Calculate the formal charges on all of the atoms except hydrogen's, in these compounds: a) H-N-N=N: c) H-C-N=N: H e) H H-=C-H b) H-N-N-N: HO: H-C-C-C 1 H d) f) H H-B-H T H
-
Calculate the formal charges on each of the atoms, except hydrogen's, of the molecules and determine the total charge of the species: a) H H-C-0_H d) H H C=N=N: b) H H-C-0: f) SF: : | H-NC-C- ...
-
Verify that the formal charges on nitrogen in ammonium ion and boron in borohydride ion are as shown.
-
There are 38 numbers in the game of roulette. They are 00, 0, 1, 2, . . ., 36. Each number has an equal chance of being selected. In the game, the winning number is found by a spin of the wheel. Say...
-
Ricky owns stock in Dove Corporation. His adjusted basis for the stock is $90,000. During the year, he receives a distribution from the corporation of $75,000 that is labeled a return of capital...
-
Determine whether the given differential equation is exact. If it is exact, solve it. (sin y - y sin x) dx + (cos x - x cos y - y) dy = 0
-
Just as in
-
Navy Surplus began July 2018 with 80 stoves that cost $10 each. During the month, the company made the following purchases at cost: The company sold 250 stoves, and at July 31, the ending inventory...
-
Materials $227,750 $195,870 Work in process 157,150 135,150 Finished goods 116,150 133,190 $409,950 437,280 Direct labor Materials purchased during January Factory overhead incurred during January:...
-
Which one of the following is an assumption that underlies cost-volume-profit analysis? 1. For costs classified as variable, the costs per unit of output must change constantly. 2. For costs...
-
Methanethiol, CH2SH, has a substantial dipole moment ( = 1.52) even though carbon and sulfur have identical electro negativities. Explain.
-
Which of the following pairs of structures represent resonanceforms? (b) :0: (a) :0: and and (d) :: (c) :0: :0: and and
-
Discuss the internal control weaknesses in the expenditure cycle flowchart for Problem 7. Structure your answer in terms of the control activities within the SAS 78/COSO control model.
-
1. List at least three (3) ways a business can anticipate potential problems to prevent complaints. 2. Explain how to identify customer needs and expectations. 3. List at least five (5) ways to build...
-
you have taken over a company with 4 employees and you have 1 millon dollars with you.as business management student,you are expected to take 5 business decisions ensuring that the company is able to...
-
Draw the Diamond - E ( SERVO ) model of strategic management and provide ONE WORD ( or short phrase ) that best describes the relationships between the elements of the model. ( up to 1 0 points )
-
If a patient's X-ray is rejected (at the end of the 22-minute evaluation by the doctor), she has a second X-ray taken (assume that the second X-ray will always be accepted) and this new X-ray must be...
-
What are the cognitive appraisal processes involved in stress perception, and how can cognitive-behavioral techniques such as cognitive restructuring and mindfulness-based interventions help...
-
Which statement is least appropriate? The next stage in the recruitment procedure is to formally define the requirements of the post. The process of setting the job description is one of considering...
-
Discuss the concept of the looking-glass self. how do you think others perceive you? do you think most people perceive you correctly?
-
The following equation of state has been proposed for a fluid where B and C are constants. a. Does this fluid exhibit a critcal point? Prove it. b. If you believe the answer to part a. is yes, derive...
-
Draw both chair conformations of 1-methyl-1-phenylcyclohexane. Which is more stable by how much energy?
-
Explain whether the methyl is axial or equatorial in this compound: CH3 H C-C-CH3 CH3
-
Draw both chair conformations for menthol (a component of peppermint oil) and its stereo isomer, neo menthol. Which groups are axial and which groups are equatorial? Explain which conformation is...
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


