Calculate the formal charges on each of the atoms, except hydrogen's, of the molecules and determine the
Question:
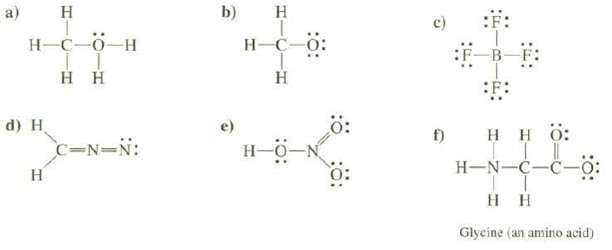
Calculate the formal charges on each of the atoms, except hydrogen's, of the molecules and determine the total charge of the species:
Transcribed Image Text:
a) H H-C-0_H Η Η d) H H C=N=N: b) H H-C-0: Η Ξ f) SF: Η Η Ο: | H-NC-C- Τ Η Η Glycine (an amino acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
The number of valence electrons is the same as the group number of the atom Unshared electron...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Calculate the formal charges on all of the atoms except hydrogen's, in these compounds: a) H-N-N=N: c) H-C-N=N: H e) H H-=C-H b) H-N-N-N: HO: H-C-C-C 1 H d) f) H H-B-H T H
-
Calculate the formal charges on the atoms shown inred. (a) (CH3)2OBF3 (b) H2C-NEN: (c) H2C=N=N: (d) :=-: (e) H (f) - CH
-
Verify that the formal charges on nitrogen in ammonium ion and boron in borohydride ion are as shown.
-
Montage Pty Limited (Montage) is a resident private company and is not a base rate entity. Which of the following transactions would result in a debit entry to Montage's franking account? Payment of...
-
Sweety Tea (ST) manufactures a range of designer T-shirts, which it produces to order for major department stores. On to March the sales department at ST received an order from retail company, Legend...
-
One of your customers is delinquent on his accounts payable balance. You've mutually agreed to a repayment schedule of $500 per month. You will charge 1.4 percent per month interest on the overdue...
-
Garcia Company issues 10%, 15-year bonds with a par value of $240,000 and semiannual interest payments. On the issue date, the annual market rate for these bonds is 8%, which implies a selling price...
-
Pure Spring Company produces premium bottled water. In the second department, the Bottling Department, conversion costs are incurred evenly throughout the bottling process, but packaging materials...
-
Practice question 6: X LLC had issued 4,000,00o equity shares of OMR 1 each. The company has the following balances. General reserves OMR 260,000 Securities premium OMR 100,00 Capital redemption...
-
The following information for the year ended December 31, 2016, was reported by Nice Bite, Inc. Required Prepare the four basic financial statements for 2016. $ 45,000 $ 17,200 Accounts Payable...
-
Using Lewis structures show a balanced equation for the reaction of H2O with HCl.
-
Predict which of the following constitutional isomers for the compound that is formed from one atom each of hydrogen, oxygen, and chlorine is more stable: H-CI-: or H-O-CI:
-
What is a page description language? Give an example of a page description language.
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Payroll Payroll Register Register Thomas Avery Towle...
-
Name: Course: Worksheet Lab Experience 5 Logic Circuits (A) Exercise 5.1 Truth table for the example circuit A B Output Value 0 0 1 1 0 1 1 Exercise 5.2 A slight change in the example circuit...
-
Stanley Medical Hospital is a non-profit and a non-chartered hospital planning to acquire several hospitals in the area. The hospital is researching financial options since they want to expand into...
-
Tony and Suzie see the need for a rugged all-terrain vehicle to transport participants and supplies. They decide to purchase a used Suburban on July 1, 2022, for $12,000. They expect to use the...
-
Pacifico Company, a US-based importer of beer and wine, purchased 1,800 cases of Oktoberfest-style beer from a German supplier for 522,000 euros. Relevant U.S. dollar exchange rates for the euro are...
-
How does job-order costing differ for service organizations and manufacturing organizations
-
DC has unused FTC carryover from 2017 in the separate category for GC income as the result of income generated by a foreign branch. The income was foreign source general category income. In 2018 the...
-
Express ln a + 1/2 ln b as a single logarithm.
-
The following model is a representation of asparatame, C14H18N2O5, known commercially as NutraSweet. Only the connection between atoms is shown; multiple bonds are not indicated. Complete the...
-
How many valence electrons does the each of the following dietary trace elements have? (a) Zinc (b) Iodine (c) Silicon (d) Iron
-
Give the ground-state electron configuration for each of the following elements: (a) Potassium (b) Arsenic (c) Aluminum (d) Germanium
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...
-
On Dec 31 2020, Bernice Melson, a partner in ABC Communications, had an ending capital balance of $49,000. Her share of the partnership's profit was $18,000; she made investments of $12,000 and had...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...

Study smarter with the SolutionInn App


