Suggest the best syntheses for each of the following ethers. Use alcohols or haloalkanes or both as
Question:
Suggest the best syntheses for each of the following ethers. Use alcohols or haloalkanes or both as your starting materials.
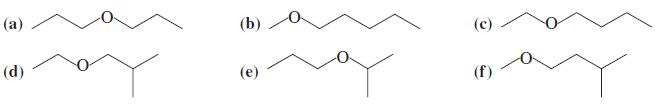
Transcribed Image Text:
(а) (b) (с) (d) (е) (f)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)

Answered By

Mallu Chenna Reddy
I completed my Ph.D. in chemistry. I have teaching experience in the subject of chemistry.
I love to help students to clear their doubts and answering to their questions.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Propose efficient syntheses for each of the following ethers, using haloalkanes or alcohols as starting materials. CH3 CH3 CH3 (a) CH3CH,CHOCH;CH; (b) OCH,CH,CH,CH3 (c) (d) CH3
-
Provide retrosynthetic analyses and syntheses for each of the following alcohols, starting with appropriate alkyl or aryl halides. (a) (b) (c) (d) (e) (f) OH (three ways) OH (three ways) (two ways)...
-
Starting with (S)-2-bromobutane, outline syntheses of each of the following compounds: (a) (b) (c) (d) (R)-CH3CHCH2CH3 OCH2CH3 (R)-CH3CHCH2CH3 CCH3 (R)-CH3CHCH2CH3 SH (R)-CH3CHCH2CH3 SCH3
-
Samson Company manufactures embroidered jackets. The company uses a standard cost system to control manufacturing costs. The following data represent the standard unit cost of a jacket: Fixed...
-
The medium-size manufacturing organization XXX Corporation has written a very complete SOW and included it in the recent RFP it sent out to prospective sellers. The project is estimated to take two...
-
Archer Company issued 4,000,000 par value, 7% convertible bonds at 99 for cash. The net present value of the debt without the conversion feature is 3,800,000. Prepare the journal entry to record the...
-
Graph the relative frequency histogram for the 500 meawrements summari~ed in the accompanying relative frequency table. Y...
-
Blackburn, Inc., an equipment manufacturer in Nashville, has submitted a sample cutoff valve to improve your manufacturing process. Your process engineering department has conducted experiments and...
-
Clawback provisions and whistleblower provisions are components of which legislation
-
Oakley Wholesale Hardware and Supplies (OWHS) sells tools, lumber, and other remodeling supplies to commercial contractors. The company controller is compiling cash and other budget Information for...
-
Suggest a good synthetic method for preparing each of the following haloalkanes from the corresponding alcohols. CH3 H3C CI I () CH-CH-CH,CI (b) CH;CH,CHCH,Br () (d) CH;CHCH(CH3)2
-
For each reaction in Problem 44, write out a detailed step-by-step mechanism. CI DMSO MP (a) CH;CH2CH,CI + CH;CH2CHCH,CH3 (b) CH3CH2CHO + CH3CH2HCH,CH3 H3C DMSO (CH:),CHOH (c) + CH3I (d) (CH3),CHO +...
-
Wind at U∞ and p∞ flows past a Quonset hut which is a half-cylinder of radius a and length L (Fig. P8.48). The internal pressure is pi. Using in viscid theory, derive an expression for...
-
In your initial post, first do the following: Use scholarly references to define Project Management (PM), Systems Development Life Cycle (SDLC), and Application Life Cycle (AL). Then, in the same...
-
How do concepts of diversity and inclusion vary across different cultural and geographical contexts, and what strategies can multinational organizations employ to navigate these variations...
-
What do you think of the gainsharing plan that Harrah's has implemented? How does an employee make more money? How much more money can they make? Is the gainsharing plan motivating employees to...
-
How do power dynamics within an organization affect employee empowerment and autonomy, and what are the best practices for creating a balanced power structure ?
-
In thinking about management and incentive structures: What recommendations do you have for the Responsible Innovation team as they seek to better embed responsible innovation within employees'...
-
Write a method called processName that accepts a Scanner for the console as a parameter and prompts the user to enter a full name, then prints the name in reverse order (i.e., last name, first name)....
-
Select a mass spectrometric technique with the highest mass resolution for identifying an unknown compound being eluted from a liquid chromatography column
-
Benzene and toluene form nearly ideal solutions. The boiling point of pure benzene is 80.1*C, Calculate the chemical potential of benzene relative to that of pure benzene when xbmzenc = 0.30 at its...
-
By measuring the equilibrium between liquid and vapour phases of a solution at 30C at 1.00 atm, it was found that xA = 0.220 when lA = 0.314. Calculate the activities and activity coefficients of...
-
Calculate the ionic strength of a solution that is 0.040 mol kg-I in K3 [Fe (CN) 6J (aq), 0.030 mol kg-1 in KCI (aq), and 0.050 mol kg3 in NaBr (aq).
-
Investors rarely hold individual securities, but rather hold collections of securities in various combinations called portfolios, typically in an effort to improve their diversification of that...
-
The following data relate to direct materials costs for February: Materials cost per yard: standard, $1.95; actual, $2.04 Standard yards per unit: standard, 4.62 yards; actual, 5.29 yards Units of...
-
finance. Can you show how they got these answers? They are correct but I am unsure on how to get it on excel or Ti 8 4 calc. Please show Suppose Ann wants to get a fully amortizing, 3 0 - year Fixed...

Study smarter with the SolutionInn App


