The mass spectra of both of the compounds described in Problem 61 show two molecular ion peaks,
Question:
The mass spectra of both of the compounds described in Problem 61 show two molecular ion peaks, two mass units apart, in an intensity ratio of about 3 : 1. Explain.
In Problem 61
Transcribed Image Text:
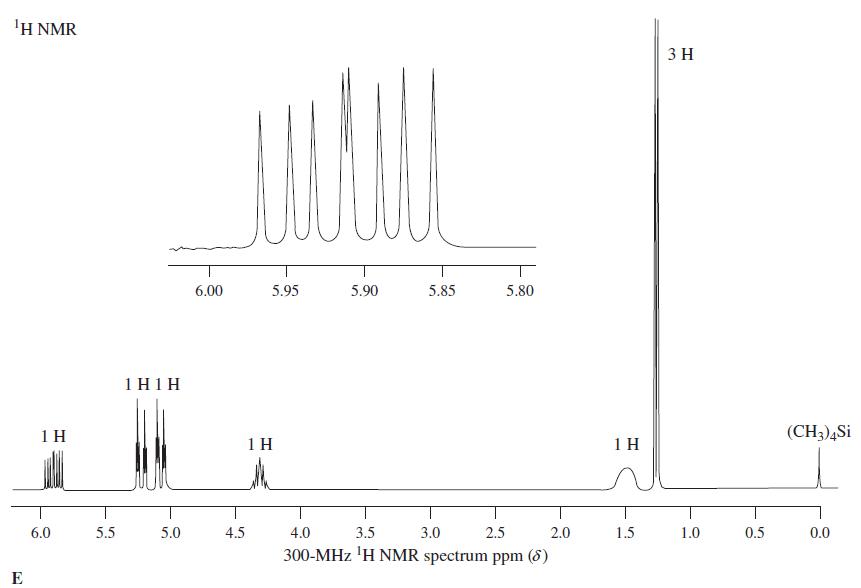
1Η ΝMR 3 H 6.00 5.95 5.90 5.85 5.80 1 H1H 1 H 1 H 1 H (CH3)4Si 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 300-MHz 'H NMR spectrum ppm (8) E
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
This suggests that the compounds have two different isotopes present in them Isotopes are atoms of t...View the full answer

Answered By

Ritul Choudhary
I am excited to introduce myself as a highly motivated and dedicated individual, who has a passion for the subject of Life Sciences. I completed my Higher Secondary education in the Montessori method of training, which has helped me to develop a unique perspective on teaching and learning. I am confident that my knowledge and skills, along with my Montessori background, make me an ideal candidate for the position. I have completed the first year of my Bachelor's degree in Life Science with a strong academic record, and I am a quick learner and have a strong ability to adapt to different situations. I am also comfortable using technology and different teaching methods to enhance the learning experience. I am looking forward to the opportunity to share my knowledge and help students achieve their full potential.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
The compounds described in Problem 27 have very different ultraviolet spectra. One has max () = 232(13,000) and 308(1450) nm, whereas the other has max () = 272(35,000) nm and a weaker absorption...
-
The 1H NMR spectra of two compounds with molecular formula C11H16 are shown here. Identify the compounds. a. b. 4 (ppm) 0 10 (ppm) frequency
-
The mass spectra of 1-methoxybutane, 2-methoxybutane, and 2-methoxy-2-methylpropane are shown in Figure 13.7. Match the compounds with the spectra. 100 73 80 S 60 57 20 0 10 20 30 40 50 60 70 80 90...
-
Which of these situations would require auditors to append an emphasis- of- matter paragraph about consistency to an otherwise unmodified opinion? a. Entity changed its estimated allowance for...
-
What is the purpose of the stakeholder communication matrix? Describe its contents.
-
True or False: SP 800-18, Guide for Developing Security Plans for Federal Information Systems,is considered the foundation for a comprehensive security blueprint and framework.
-
Company cars are commonly provided by UK employers for senior staff. The tax regime introduced in 2002 aimed to discourage demand for larger cars which are not environmentally friendly. LO4
-
At the beginning of 2007 Ace Company had the following portfolio of investments in available-for-sale securities (common stock): During 2007 the following transactions occurred: May 3 Purchased C...
-
An investor is concerned about interest rate risk. Which of the following four bonds (simiar except for yield and maturity) has the MOST interest rate risk? The bond with: A. 6% yield and 10-year...
-
The following data report total, monthly U.S. book-store sales in millions of dollars fromJanuary 2016 to March 2019. (Go to https://www.census.gov/retail/index.html#mrts, find Monthly Retail Trade...
-
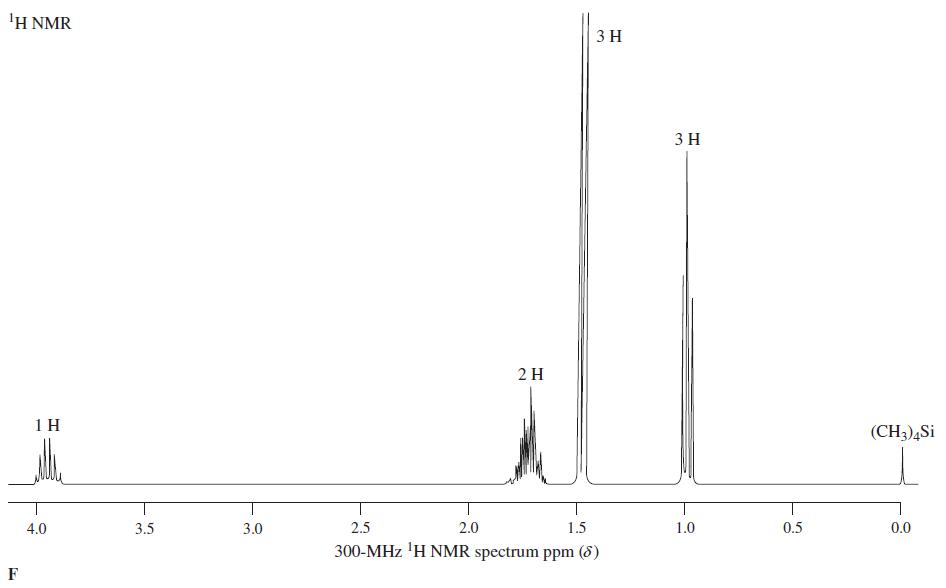
Reaction of the compound corresponding to spectrum E with SOCl 2 produces a chloroalkane, C 4 H 7 Cl, whose NMR spectrum is almost identical with spectrum E, except that the broad signal at = 1.5...
-
Give the structure of an alkene that will give the following carbonyl compounds upon ozonolysis followed by reduction with (CH 3 ) 2 S. (a) CH 3 CHO only (b) CH 3 CHO and CH 3 CH 2 CHO (c) (CH 3 ) 2...
-
Define each of the following terms: a. Working capital; net working capital; net operating working capital b. Inventory conversion period; receivables collection period; payables deferral period;...
-
Change the session date to July 8, 2024. Create shortcuts or change modules and enter the following transactions. NOTE: Deposits and withdrawals, except credit card transactions, use Bank: Chequing...
-
Determine the intervals on which the function f(x) = 1 x + 2/3 4 3 -x - 1 2 - -2x is increasing and the intervals on which it is decreasing. f(x) is increasing for x = (-2, -1) U (1,) and decreasing...
-
Read the buret (burette) volume and report your reading with the proper number of digits. Number 3.2 mL mL 0 10 15 46 20 25 30 35 47 40 48 Incorrect.
-
TCP Congestion Control using Wireshark and testmy.net. Identify the IP Address, Protocol (UDP or TCP), Destination and Source IP Address, and IP Class Type (A-D).
-
Charley & Waldo's World of Wonder is a science-oriented children's museum. The museum has a "free" section where children have unlimited use science oriented exhibits and a premium section where...
-
Write code to examine a String and determine how many of its letters come from the second half of the alphabet (that is, have values of 'n' or subsequent letters). Compare case-insensitively, such...
-
Read the Forecasting Supply Chain Demand Starbucks Corporation case in your text Operations and Supply Chain Management on pages 484-485, then address the four questions associated with the...
-
A laser used to read CDs emits red light of wavelength 700 nm. How many photons does it emit each second if its power is? (a) 0.10 W, (b) LOW?
-
The work function for metallic cesium is 2.14 eV. Calculate the kinetic energy and the speed of the electrons ejected by light of wavelength (a) 700 nm, (b) 300 nm.
-
Calculate the size of the quantum involved in the excitation of (a) An electronic oscillation of period 2.50 fs, (b) A molecular vibration of period 2.21 fs, (c) A balance wheel of period 1.0 ms....
-
Akuse Tours company secures a loan of GHS200,000 over 2 years at 10.5% compounded quarterly year to purchase more tour buses for the upcoming Christmas festivities. Requirements: a. Compute the...
-
The fifth step of the interview process is: Set expectations Review the results Handle client issues and questions
-
An individual recently won a lottery and has the opportunity to receive $100,000 per year at the end of the year for the next 25 years. Assuming an annual interest rate of 5% is appropriate, the...

Study smarter with the SolutionInn App


