Give the structure of an alkene that will give the following carbonyl compounds upon ozonolysis followed by
Question:
Give the structure of an alkene that will give the following carbonyl compounds upon ozonolysis
followed by reduction with (CH3)2S.
(a) CH3CHO only
(b) CH3CHO and CH3CH2CHO
(c) (CH3)2C P O and H2C P O
(d)
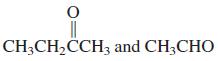
(e) Cyclopentanone and CH3CH2CHO
Transcribed Image Text:
CH3CH,CCH3 and CH3CHO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
The solutions of the above questions are as follows Ozone lysis is an organic reaction where th...View the full answer

Answered By

Yadram Dhanka
I was engaged in conducting private tuitions for students of class 11th and 12th. I would like to work with a leading educational organization and to use my in-depth subject knowledge and passion towards teaching to the best of my ability, so as to enrich the student’s ability to learn, as well as to advance my career in the education sector.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Draw the structure of an alkene that yields only acetone, (CH3)2C = O, on ozonolysis followed by treatment with Zn.
-
For each of the following cases, provide the structure of an alkene that would give the alcohol as the major (or only) product of hydroboration--oxidation. CH CH CH CH OH CH, CH,
-
Give the structure of a compound with the indicated formula that would give the following diol in a LiAlH4 reduction followed by protonolysis. C8H6O4 HOCH,- -CH,OH
-
With the company expanding into several new markets in the coming months, Cable & Moore was anticipating a large increase in sales revenue. The future looked bright for this provider of...
-
The eight quality management principles follow:
-
Mintz Industries has sales in 2012 of $5,600,000 (800,000 units) and gross profit of $1,344,000. Management is considering two alternative budget plans to increase its gross profit in 2013. Plan A...
-
I:5-57 Original Issue Discount. On January 1, 2021, Sean purchased an 8%, $100,000 corporate bond for $92,277. The bond was issued on January 1, 2021, and matures on January 1, 2026. Interest is paid...
-
Pine Products Company uses a job order cost system. For a number of months there has been an ongoing rift between the sales department and the production department concerning a special-order...
-
Flexibility of practice when applied to managerial accounting means that:
-
Ombese owns and manages a small manufacturing business. The following balances have been extracted from her books of accounts at 31 December 2021. Dr. Purchase of Raw materials Fuel and Light...
-
The mass spectra of both of the compounds described in Problem 61 show two molecular ion peaks, two mass units apart, in an intensity ratio of about 3 : 1. Explain. In Problem 61 1 MR 3 H 6.00 5.95...
-
Plan syntheses of each of the following compounds, utilizing retrosynthetic-analysis techniques. Starting compounds are given in parentheses. However, other simple alkanes or alkenes also may be...
-
Mason Enterprises' net marketing contribution of $50 million is derived from sales of $200 million. If its marketing and sales expenses amount to $20 million, what is its percentage of gross profit...
-
Let f(x) In(x). Solve each of the following equations exactly for a. (f(x)) = 11 b. f(x) = 11 c. f(x) = 11
-
Suppose the annual rate of inflation in Taiwan is 6.66%, and the annual rate of inflation in Mexico is 5.99%. If the Mexican peso depreciates relative to the Taiwan dollar by 4% in real terms, then...
-
What is the average age (measured by the variable "age") of the sample in the GSS93 subset.sav data set? Is there a significant difference in the age of those who favor the death penalty for murder...
-
Solve the system of linear equations, using the Gauss-Jordan elimination method. (If there is no solution, enter NO SOLUTION. If there are infinitely many solutions, express your answer in terms of...
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Consider a method printTriangleType that accepts three integer arguments representing the lengths of the sides of a triangle and prints the type of triangle that these sides form. The three types are...
-
What is the expected payoff of an investment that yields $5,000 with a probability of 0.15 and $500 with a probability of 0.85? Select one: O a. $325 O b. $5,500 O c. $2,750 O d. $1,175
-
Calculate the de Broglie wavelength of an electron accelerated from rest through a potential difference of (a) 100 V, (b) 1.0 kV, (c) 100 kV.
-
Show that the linear combinations A + iB and A - iB are not hermitian if A and E are hermitian operators.
-
An electron is confined to a linear region with a length of the same order as the diameter of an atom (about 100 pm). Calculate the minimum uncertainties in its position and speed.
-
Suarez Foods has a bond issue with a face value of $20,000 that is coming due in one year. The current value of the firm's assets is $22,200 but these assets are expected to be worth either $18,000...
-
REITs can expand their income by: Group of answer choices Speculating with build-to-suit projects for existing tenants By engaging in any or all of these activities Offering property management,...
-
Mercury, Incorporated, produces cell phones at its plant in Texas. A year ago, a consumer survey ranked the company s cell phones low in product quality. Shocked by this result, Jorge Gomez, Mercury...

Study smarter with the SolutionInn App


