For each of the following cases, provide the structure of an alkene that would give the alcohol
Question:
For each of the following cases, provide the structure of an alkene that would give the alcohol as the major (or only) product of hydroboration--oxidation.
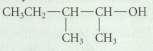
Transcribed Image Text:
CH CH CH CH OH CH, CH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
CHCHC CHCH3 C...View the full answer

Answered By

Rashul Chutani
I have been associated with the area of Computer Science for long. At my university, I have taught students various Computer Science Courses like Data Structures, Algorithms, Theory of Computation, Digital Logic, System Design, and Machine Learning. I also write answers to questions posted by students in the area of and around Computer Science.
I am highly fortunate to receive great feedback on my teaching skills that keeps me motivated. Once a student sent me an email stating that I had explained to him a concept better than his professor did.
I believe in the fact that "Teaching is the best way to learn". I am highly fascinated by the way technology nowadays is solving real-world problems and try to contribute my bit to the same.
Besides tutoring, I am a researcher at the Indian Institute of Technology. My present works are in the area of Text Summarization and Signal and Systems.
Some of my achievements include clearing JEE Advanced with an All India Rank of 306 out of 1.5 million contesting candidates and being the Department Ranker 1 at my University in the Department of Computer Science and Engineering.
I look forward to providing the best Tutoring Experience I can, to the student I teach.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Provide the reagents necessary for each of the following transformations. In some cases several steps may be necessary. (c) (d) (e) (f)
-
For each of the following cases, give the product(s) of the transformation inducted by the curved - arrow notation. (a) (b) (CHs)2C-C(CH3)2 H-Br: O: O: -: 0 :
-
Provide a structure for each of the following compounds. C 9 H 10 O 3 : IR 2400-3200, 1700, 1630 cm 1 NMR: 1.53 (3H, t, J = 8 Hz); 4.32 (2H, q, J = 8 Hz); 7.08, 8.13 (4H, pair of leaning...
-
Use a calculator to express each number in Problems 39 and 40 as a decimal to the capacity of your calculator. Observe the repeating decimal representation of the rational numbers and the non...
-
What verbal and/or nonverbal communication "Rules" or "Norms" would a person advocate changing to promote more effective communication within and across the different areas of their life?
-
\(f(x)=x^{3}\) Evaluate the functions at the values \(f(-2), f(-1), f(0), f(1)\), and \(f(2)\).
-
8. Under what circumstances would the direction of intercompany inventory transactions not affect the allocation of unrealized profit?
-
McKeekin Corp. has a project with the following cash flows: Year Cash Flow 0 ..................... $20,000 1 ...................... -26,000 2 ....................... 13,000 What is the IRR of the...
-
How much would you have to invest today to receive: (Use a Financial calculator to arrive at the answers. Round the final answers to the nearest whole dollar.) a. $16,500 in 7 years at 11 percent?...
-
On January 1, 2016, Theta Corp. acquired a four unit apartment building at as cost of $1,475,000. Of this total, it is estimated that the land on which the building is situated is worth $250,000 and...
-
Iodine azide,I-Nr, adds to isobutylene in the following manner: iodine azide
-
Which of the following alkenes would yield the same alcohol from either oxymercuration-reduction or hydroboration--oxidation, and which would give different alcohols? Explain. (a) cis-2-butene (b)...
-
The smallest possible piece of the compound hydrogen peroxide contains two hydrogen atoms and two oxygen atoms. Write the chemical formula for hydrogen peroxide, with hydrogen first.
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28W / (m ^ 2) ."C. The thermal conductivity of the solid...
-
Which of the following best demonstrates the Six Cs of Communication, "you" approach, and positive emphasis? Question 1 4 options: It will be February 1 0 before you will receive your materials. It...
-
please answer all the questionss.,.within 30 minutes. make sure the explanation and reasons are explained in very detailed manner as in why the chosen option is right and why other options are wrong....
-
1) A net force of 20 N is applied to the right on an object. If the acceleration of the object is 2.5 m/sec, a) What is the mass of the object? (8 kg) b) What is the weight of the object? (78.4 N) c)...
-
BO Corp. has a $2,500 capital budget, and has access to the following 5 independent projects. In all these 5 projects, cash outflows occur only in year O. Calculate the total NPV of the project(s)...
-
Explain the difference between the total PMPM and a premium rate.
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
In each of the following parts, explain why the first compound has a higher boiling point than the second, despite a lower molecular mass. (a) (b) HC-C-OH (bp 118 C) O H3C-C-NH (bp 221 C) HC-C-OCHCH3...
-
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each case. (a) With which one of the following solvents is DMSO not miscible: water, acetone,...
-
Give an IUPAC name for each of the following compounds, which may have been isolated from the shoes of a tennis player. Ignore stereochemistry in (a). (a) (b) HO
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


