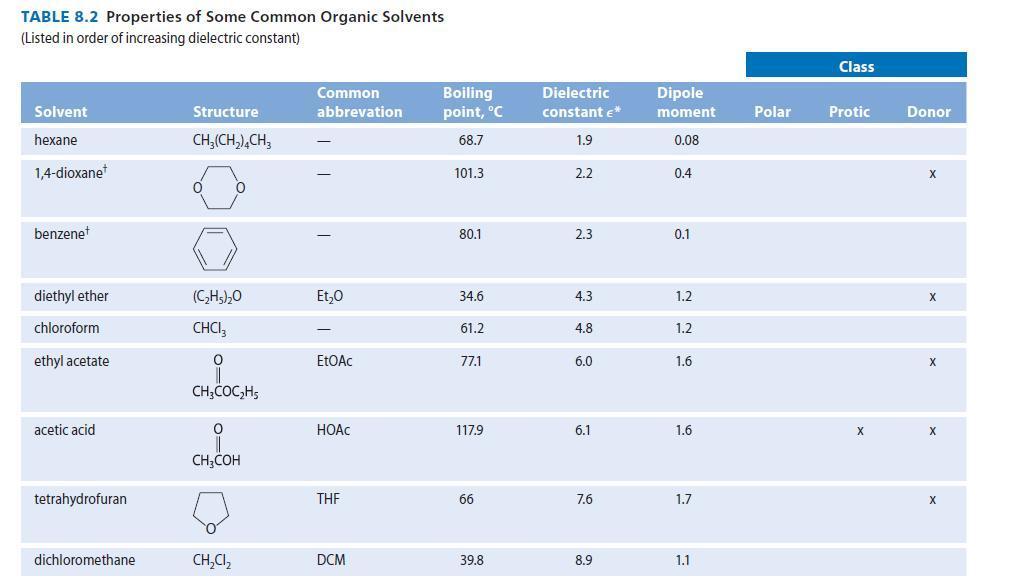
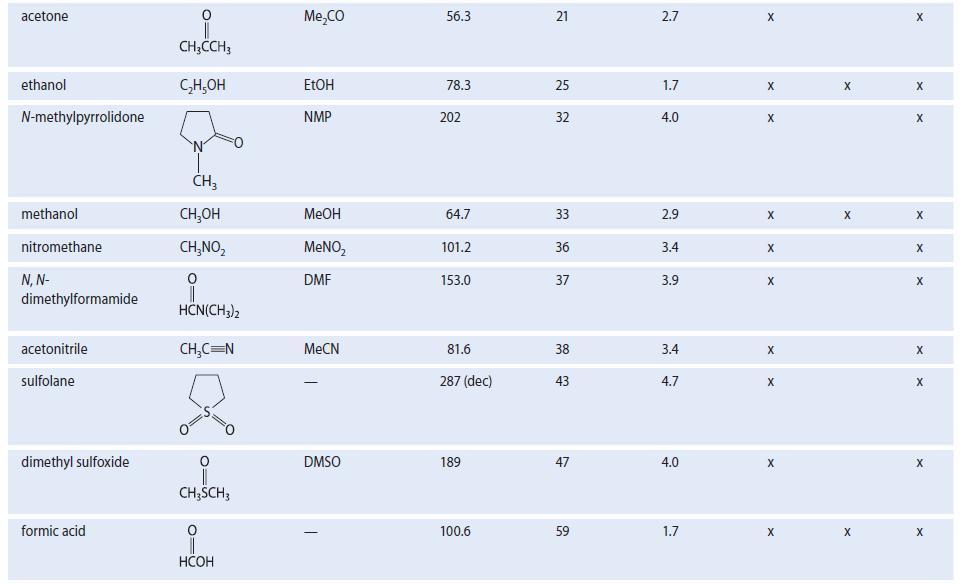
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each
Question:
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each case.
(a) With which one of the following solvents is DMSO not miscible: water, acetone, hexane, or acetonitrile?
(b) With which one of the following solvents is hexane not miscible: methanol, 1-propanol, diethyl ether, or acetone?

Transcribed Image Text:
TABLE 8.2 Properties of Some Common Organic Solvents (Listed in order of increasing dielectric constant) Solvent hexane 1,4-dioxane benzenet diethyl ether chloroform ethyl acetate acetic acid tetrahydrofuran dichloromethane Structure CH₂(CH₂), CH3 0 (C₂H5)₂0 CHCI 0 CH3COC₂H5 0 CH3COH CH₂Cl₂ Common abbrevation Et₂0 EtOAc HOAC THE DCM Boiling point, °C 68.7 101.3 80.1 34.6 61.2 77.1 117.9 66 39.8 Dielectric constant €* 1.9 2.2 2.3 4.3 4.8 6.0 6.1 7.6 8.9 Dipole moment 0.08 0.4 0.1 1.2 1.2 1.6 1.6 1.7 1.1 Polar Class Protic X Donor X X X X X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The ability of solvents to mix with each other miscibility is largely dependent on the similarity of their polarity and ability to engage in hydrogen ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which would you expect to be the stronger acid? Explain your reasoning in each instance. (a) CH2ClCO2H or CHCl2CO2H (b) CCl3CO2H or CHCl2CO2H (c) CH2FCO2H or CH2BrCO2H (d) CH2FCO2H or CH2FCH2CO2H
-
The file S02_35.xlsx contains (fictional) data from a survey of 500 randomly selected households. a. Indicate the type of data for each of the variables included in the survey. b. For each of the...
-
The auditor uses MUS to select accounts receivables for confirmation. In confirming individual accounts receivable balances, your client's customers reported the exceptions listed below. Required...
-
Lacoste t-shirts come with an average price of $ 120 a piece, at their factory outlet with a std. deviation of $ 17. But at the Seasonal Sale (Discount) outlets of these t- shirts, it was also...
-
The court states that the whole purpose of a letter of credit would be defeated by examining the merits of the underlying contract. What does that mean?
-
A simply supported beam ABC is loaded by a vertical load P acting at the end of a bracket BDE (see figure). Draw the shear-force and bending-moment diagrams for beam ABC. IeI 2 4 4
-
Explain identifying and working with foreign intermediaries. 1239
-
Kim Ries, Tere Bax, and Josh Thomas invested $40,000, $56,000, and $64,000, respectively, in a partnership. During its first calendar year, the firm earned $124,500. Required Prepare the entry to...
-
there are no questions Input area: Year: Year: Cost of goods sold Cash Depreciation Interest expense Selling & Administrative Accounts payable Net Fixed assets Sales Accounts receivable Notes payable...
-
In each of the following parts, explain why the first compound has a higher boiling point than the second, despite a lower molecular mass. (a) (b) HC-C-OH (bp 118 C) O H3C-C-NH (bp 221 C) HC-C-OCHCH3...
-
Give an IUPAC name for each of the following compounds, which may have been isolated from the shoes of a tennis player. Ignore stereochemistry in (a). (a) (b) HO
-
The equation mgy for gravitational potential energy is valid only for objects near the surface of a planet. Consider two very large objects of mass m 1 and m 2 , such as stars or planets, whose...
-
This case study is based on a fictional character on NBC's The Office. Michael is the central character of the series, serving as the Regional Manager of the Scranton branch of a paper distribution...
-
What is the significance of a balance sheet in understanding a firm's financial position? How do changes on the right side of the balance sheet (liabilities and equity) impact a company's financial...
-
A current event analysis where the article must focus on a management concepts). You will read the article and then provide an analysis of the subject matter discussed. The article should complement...
-
Given an exponential distribution with =20, what is the probability that the arrival time is a. less than X=0.2? b. greater than X = 0.2? c. between X=0.2 and X 0.3? d. less than X=0.2 or greater...
-
Choose at least two measures of employee attitudes. Discuss them and tell me about your discussion. Which group you believe are the most effective and efficient measures? Why? 2) Discuss turnover,...
-
Name the layers of the meninges, and explain their functions.
-
Suppose that a business sells 6-month subscriptions to its monthly magazine. On January 1, the company receives a total of $600 for 10 subscriptions. To record this transaction, the company debits...
-
Explain whether each compound is soluble in aqueous NaOH, aqueous NaHCO3 both, or neither.
-
Calculate these quantities: (a) The wavelength of light (in centimeters) with a frequency of 9.00 x 1012Hz. (b) The frequency of light with a wavelength of 310nm (c) The energy of light (in kcal/mol...
-
What kind of light has a frequency of 9.00 x 1013Hz?
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


