In each of the following parts, explain why the first compound has a higher boiling point than
Question:
In each of the following parts, explain why the first compound has a higher boiling point than the second, despite a lower molecular mass.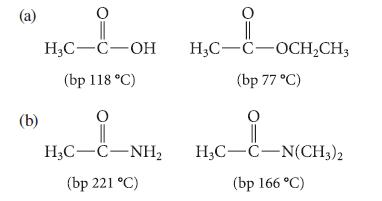
Transcribed Image Text:
(a) (b) H₂C-C-OH (bp 118 °C) O H3C-C-NH₂ (bp 221 °C) H₂C-C-OCH₂CH3 (bp 77 °C) O H3C-C-N(CH3)2 (bp 166 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Explanation Acetic acid CH3COOH has higher boiling point 118 C than ethyl acetate CH3COOCH2CH3 bp ...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each of the following parts. Explain why the first compound has a higher boiling pc-rint than the second, despite a lower molecular mass. (bp 221 C) (bp 166 C)
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Explain why a. H2O has a higher boiling point than CH3OH(65oC). b. H2O has a higher boiling point than NH3(- 33oC). c. H2O has a higher boiling point than HF (20C).
-
Do you agree or not? Specialization of labor and better use of capital goods can initially generate increasing marginal output (returns) for a firm in the production of a good.
-
Was the Calcu-Folio properly classified as a briefcase or a binder?
-
The cantilever beam AB shown in the figure is subjected to a concentrated load P at the midpoint and a counterclockwise couple of moment M1 = PL/4 at the free end. Draw the shear-force and...
-
Describe how to manage export-import transactions. 1239
-
Greene Sisters has a DSO of 20 days. The companys average daily sales are $20,000. What is the level of its accounts receivable? Assume there are 365 days in a year.
-
2. Consider a corporate bond with a coupon rate of 5%, a maturity of 4 years, and an interest payment every six months. The price is 97.50. The spot interest rate is as follows: Table 2-2 Period Spot...
-
Without consulting tables, arrange the compounds within each of the following sets in order of increasing boiling point, and give your reasoning. (a) 1-hexanol, 2-pentanol, tert-butyl alcohol (b)...
-
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each case. (a) With which one of the following solvents is DMSO not miscible: water, acetone,...
-
(a) Show that every member of the family of functions y = Cex2/2 is a solution of the differential equation yt = xy. (b) Illustrate part (a) by graphing several members of the family of solutions on...
-
1. What are the threats being faced by Indian General Insurance Ltd. (IGIL)? 2. What are its traditional strengths? What 'business definitions' should it follow while capitalizing on its traditional...
-
You go to discuss the incident and the client's claims with your supervisor. As you retell the incident, it is clear that your supervisor is not comfortable. You ask your supervisor for advice on the...
-
Case Study Two: Rawlings Rawlings is an American sports equipment manufacturing company based in Town and Country, Missouri, and founded in 1887. Rawings specializes in baseball equipment and...
-
The discussion is for Administrating organizational change course. (we should write 300 words) Discussion question is: Refer to table 6.4 in your book. Think of a time when you were introduced to...
-
Content: Identify at least two resources for each of the four critical sections in the course project: Strategic Planning, Healthcare Reimbursement, Revenue Cycle Process, and Reimbursement...
-
Name the functions of the midbrain, pons, and medulla oblongata.
-
Time Travel Publishing was recently organized. The company issued common stock to an attorney who provided legal services worth $25,000 to help organize the corporation. Time Travel also issued...
-
Name these compounds. Discuss.
-
Explain which compound has the higher melting point.
-
Explain which compound has the higher boiling point. Discuss in detail.
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


