In each of the following parts. Explain why the first compound has a higher boiling pc-rint than
Question:
In each of the following parts. Explain why the first compound has a higher boiling pc-rint than the second, despite a lower molecular mass.
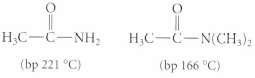
Transcribed Image Text:
(bp 221 °C) (bp 166 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
The NH hydrogens of the first compound acetamide can be donated in hydrogen bonds ...View the full answer

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In each of the following pairs, which compound would you expect to have the higher standard molar entropy: (a) C2H2(g) or C2H6(g) (b) CO2(g) or CO(g)? Explain.
-
In each of the following pairs, indicate which has the higher concentration of I- ion:
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
An astronaut must journey to a distant planet, which is 200 light-years from Earth. What speed will be necessary if the astronaut wishes to age only 10 years during the round trip?
-
Commercial Recording, Inc., is a manufacturer and distributor of reel-to-reel recording decks for commercial recording studios. Revenue and cost relations are: TR = $3,00- $00Q.5Q2 AND TC = $100,000...
-
Euphonium Ltd. has an opportunity to obtain a new contract for the production of a new valve. The valve requires 200 hours of processing on machine A, which is already working at full capacity on the...
-
is the differentiation strategy only appropriate for luxury goods? Explain.LO1
-
Gosnell Company produces two products: squares and circles. The projected income for the coming year, segmented by product line, follows: The selling prices are $30 for squares and $50 for circles....
-
plzzz help T $1,200 plus GST Question 1 (30 Marks) Star delivery Pty Ltd provides delivery services in NSW rural areas. The unadjusted trial balance on 30 June 2017 below was prepared by the...
-
The G. Saussy Manufacturing company is putting out four new electronic components. Each of Saussy's four plants has the capacity to add one more product to its current line of electronic parts. The...
-
Withc-rut consulting tables, arrange the compounds within each of the following sets in order of increasing boiling point, and give your reasoning. (a) I -hexanol, 2-pentanol, tert-butr,l alcohol (b)...
-
Give a structure for each of the following compounds. (In some cases, more then one answer is possible) (a) A chiral alcohol C4H6O (b) A diol C4H10O2, that exists in only three stereoiso meric forms
-
Your friend, Wendy, plans to open a hair salon. Wendy states that she does not have time to develop and implement a system of internal controls. a. Explain to Wendy the objectives of a system of...
-
Below are listed some additional common performance measures not listed in Exhibit 2.1. Which type of employee (senior managers, middle managers, or frontline operations managers) would typically use...
-
If you have a steam distillation system with immiscible organic and water phases plus a vapor phase, two volatile organic compounds plus a nonvolatile organic compound, at equilibrium how many...
-
An auditor is using difference estimation for the confirmation of accounts receivable in the audit of Lafferty Hardware Supply. A random sample of 100 positive confirmations has been sent to...
-
Canterbury Convenience Stores (CCS) is a newly formed organization in Christchurch, New Zealand. It comprises 10 moderately sized convenience stores that previously operated independently of each...
-
Orchard Distributions Pte. Ltd. is a large, Singaporean-based distributor of clothing products to other companies throughout Southeast Asia. Orders are received from customers either by telephone,...
-
Should managers of investor-owned providers focus exclusively on a project's market risk?
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
Give the principal product(s) expected, if any, when trans-1,3-pentadiene reacts under the following conditions. Assume one equivalent of each reagent reacts unless noted otherwise. (a) Br 2 (dark)...
-
Using the Hckel 4n + 2 rule, determine whether each of the following compounds is likely to be aromatic. Explain how you arrived at the -electron count in each case. (a) (c) CO (b) -C=C- (d) :1
-
The following molecule has a barrel shape (in which the benzene rings are the walls of the barrel). It forms a noncovalent complex with the iodide salt of acetylcholine in chloroform solvent....
-
Analysis of a replacement project At times firms will need to decide if they want to continue to use their current equipment or replace the equipment with newer equipment. In this case, the company...
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...
-
Q3. Company ABC has accounting income $500 for year 2016, 2017 and 2018, with following balance 2015 2016 2017 2018 Accounts Payable 100 110 120 90 Unearned Revenue 100 50 30 0 Prepaid Expense 100 80...

Study smarter with the SolutionInn App


