The following molecule has a barrel shape (in which the benzene rings are the walls of the
Question:
The following molecule has a barrel shape (in which the benzene rings are the “walls” of the barrel). It forms a noncovalent complex with the iodide salt of acetylcholine in chloroform solvent.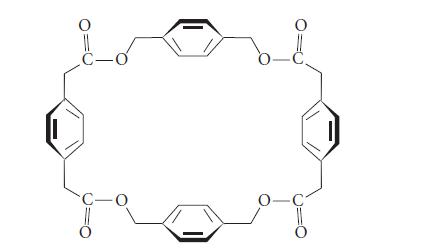
Describe the orientation of the acetylcholine molecule within the complex.
Transcribed Image Text:
O C-o C-O 8 O -C₂ 0-C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The positive end of the acetylcholine ion would orient its...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The N-methylquinolinium ion forms a noncovalent complex with molecule A in water that has a standard free energy of dissociation G d 5 28.9 kJ mol 1 (6.9 kcal mol 1 ). The neutral molecule...
-
Identify the process evaluation article that you chose and explain why you selected this example. Describe the purpose of the evaluation, the informants, the questions asked, and the results of the...
-
Which choice is greener in a chemical process? Explain. (a) Benzene as a solvent or water as a solvent. (b) The reaction temperature is 500 K, or 1000 K. (c) Sodium chloride as a by-product or...
-
What are some Marketing Strategies for Delivering Objectives Polestar (Car company) has used or using?
-
What is the maximum penalty and prison term that can be charged to a CEO and/or CFO under the Sarbanes-Oxley Act?
-
1. How has Cisco changed its structure and control systems? 2. Relate Cisco's changes to its control and evaluation systems to the stages of growth in Greiner's model. 3. Use the Internet to...
-
Extending the life of an aluminum smelter pot. An investigation of the properties of bricks used to line aluminum smelter pots was published in The American Ceramic Society Bulletin (Feb. 2005). Six...
-
A shop sign weighing 245 N is supported by a uniform 155-N beam as shown in Fig. 9-54. Find the tension in the guy wire and the horizontal and vertical forces exerted by the hinge on the beam. 35.0...
-
Several types of financial opinions healthcare organizations have access to finance projects.
-
Using the Hckel 4n + 2 rule, determine whether each of the following compounds is likely to be aromatic. Explain how you arrived at the -electron count in each case. (a) (c) CO (b) -C=C- (d) :1
-
Which of the compounds or ions in Problem 15.38 are likely to be antiaromatic? Explain. Problem 15.38 Which of the following species should be aromatic by the Hckel 4n + 2 rule? (a) thiophene G O H H...
-
Review Conceptual Example 7 in preparation for this problem. In tests on earth a lunar surface exploration vehicle (mass = 5.90 10 3 kg) achieves a forward acceleration of 0.220 m/s 2 . To achieve...
-
1.1 Indonesia is it potential as a market for Apple? 2.1 Examination of Apple's entry strategy into the international market? 2.2 Evaluation of the entry mode(s) employed by Apple and their...
-
Dynamic, a global media agency, has recently taken over MediaHype, a local agency in Melbourne, to expand its Australian operations. Jeff Tan, a Chinese national, has been appointed to head the new...
-
Linear optimization models play a crucial role in improving supply chain management efficiency, both in physical and abstract network problems. Three ways they can be applied are through optimizing...
-
When I consider optimizing the portfolio allocation for both my 403(b) and CALSTRS retirement accounts, I find it crucial to employ a well-structured model to ensure that my investments align with my...
-
How can you use your understanding of diversity to develop your relationship-building skills in your healthcare career?,Explain ways in which religion can help or hinder individuals as they build...
-
Find a function whose derivative is (a) 2x (b) sin x (c) x2 + x + 1
-
Calculate the number of neutrons of 239Pu.
-
Propose structures for compounds that meet the following descriptions: (a) C5H8, with IR absorptions at 3300 and 2150 cm1 (b) C4H80, with a strong IR absorption at 3400 cm1 (c) C4H80, with a strong...
-
How could you use infrared spectroscopy to distinguish between the following pairs of isomers? (a) HC CCH2NH2 and CH3CH2C N (b) CH3COCH3 and CH3CH2CHO
-
Two infrared spectra are shown. One is the spectrum of cyclohexane, and the other is the spectrum of cyclohexane. Identify them, and explain youranswer. (a) 100 80 60 20 - 1000 4000 3500 3000 2500...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


