Using the Hckel 4n + 2 rule, determine whether each of the following compounds is likely to
Question:
Using the Hückel 4n + 2 rule, determine whether each of the following compounds is likely to be aromatic. Explain how you arrived at the π-electron count in each case.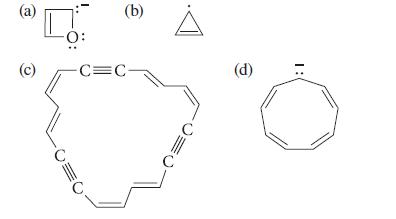
Transcribed Image Text:
(a) (c) CO (b) -C=C- (d) :1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a b c d This species contains six 7 electrons two from the double bond two from ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the values of the diffusion coefficients for the interdiffusion of carbon in both iron (BCC) and iron (FCC) at 900C Which is larger Explain why this is the case
-
Using the Huckel 4n + 2 rule, determine whether each of the following compounds is likely to be aromatic. Explain how you arrived at the -electron count in each case. (a) (b)
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Research is a process of discovering new knowledge. In the Code of Federal Regulations (45 CFR 46.102(d)) pertaining to the protection ofhuman subjects,research is defined as:...
-
What are the six articles of ethical conduct set out under section I of the AICPAs Code of Professional Conduct?
-
When would a company choose a matrix structure? What are the problems associated with managing this structure, and why might a product-team structure be preferable?
-
New method of estimating rainfall. Accurate measurements of rainfall are critical for many hydrological and meteorological projects. Two standard methods of monitoring rainfall use rain gauges and...
-
Refer to the data provided in E4A. In E4A. In chronological order, the inventory, purchases, and sales of a single product for a recent month are as follows. 1. Using the perpetual inventory system,...
-
2. Ling has saved $68,200 00 If she decides to withdraw $2,626.00 at the beginning of every month and interest is 10.42% compounded annually, for how long can she make withdrawals? Express your...
-
Give the principal product(s) expected, if any, when trans-1,3-pentadiene reacts under the following conditions. Assume one equivalent of each reagent reacts unless noted otherwise. (a) Br 2 (dark)...
-
The following molecule has a barrel shape (in which the benzene rings are the walls of the barrel). It forms a noncovalent complex with the iodide salt of acetylcholine in chloroform solvent....
-
What is the voltage across six 1.5-V batteries when they are connected (a) In series, (b) In parallel, (c) Three in parallel with one another and this combination wired in series with the remaining...
-
The Log Jamboree amusement park ride at Six Flags over Georgia consists of an approximately rectangular flume that is 6 ft wide and is constructed from fiberglass (ks = 0.002 in). In the low-velocity...
-
57'-8" 1. The building perimeter walls are 1'2" thick and the interior walls are 1'0" thick. Fig 1 and Fig 2 detail the linear feet of 1'2" -thick foundation walls. In addition, side B is 8'4" tall...
-
The Orpheus Chamber Orchestra is celebrating its 50 years as an orchestra this year. Read the following articles about its unique structure: The first Charlotte article is copied below, the rest just...
-
Which of the five strategies for adapting products and promotion for global markets does Monster Employ? 15-16. Which factors in the global marketing environment have challenged Monster's global...
-
Analysis of Current International Economic Environment in Switzerland 1. Develop a lead sentence for this section that introduces the key subsections 2. Economic Environment describe Switzerland...
-
Add 1 to each answer from Problem 21. Are these functions also solutions to Problem 21? Explain.
-
Define the essential properties of the following types of operating systems: a. Batch b. Interactive c. Time sharing d. Real time e. Network f. Parallel g. Distributed h. Clustered i. Handheld
-
How might you use IR spectroscopy to distinguish among the three isomers 1 -butyne, 1, 3-hutadiene, arid 2-butyne?
-
Would you expect two enantiomers such as (R)-2-brornobutane and S)-2-bromobutane to have identical or different IR spectra explain.
-
Would you expect two diastereomers such as meso-2, 3-dibromobutanc and (2R, 3R)-dibromo butane to have identical or different IR spectra? Explain.
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


