Give an IUPAC name for each of the following compounds, which may have been isolated from the
Question:
Give an IUPAC name for each of the following compounds, which may have been isolated from the shoes of a tennis player. Ignore stereochemistry in (a).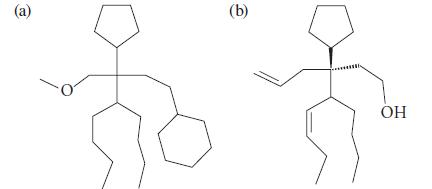
Transcribed Image Text:
(a) (b) HO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The IUPAC names for the compounds in the image are Compound a 2hydroxy2methylpropanoic acid also kno...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the IUPAC name for each of the following compounds: (a) CH3(CH2)25CH3 (b) (CH3)2CHCH2(CH2)14CH3 (c) (CH3CH2)3CCH(CH2CH3)2 (d) (e) (f) (g)
-
Give the IUPAC name for each of the following alkyl groups, and classify each one as primary, secondary, or tertiary: (a) CH3(CH2)10CH2-- (b) (c) --C(CH2CH3)3 (d) (e) (f) -CH2CH2CHCH2CH2CH3 CH2CH3...
-
Give the IUPAC name for each of the following compounds. a. b. CH C-CHCHCH CH CH2 CH3 CH CH2CH2CHCH2CH2CH3 CH CH2
-
You are the CEO of Green Paper Inc., a producer of high-end printing paper with an emphasis on environmentally friendly "green" production methods. One of your employees has proposed a significant...
-
Why is a confirmed LOC even more attractive to a seller than one that is not confirmed?
-
A beam ABC is simply supported at A and B and has an overhang BC (see figure). The beam is loaded by two forces P and a clockwise couple of moment Pa that act through the arrangement shown. Draw the...
-
Understand outsourcing, global sourcing, and offshoring. 1239
-
Venus Candy Company budgeted the following costs for anticipated production forSeptember 2010: Prepare a factory overhead cost budget, separating variable and fixed costs. Assume that factory...
-
On December 3 1 , 2 0 2 3 , Berclair Incorporated had 5 0 0 million shares of common stock and 2 3 million shares of 9 % , $ 1 0 0 par value cumulative preferred stock issued and outstanding. On...
-
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each case. (a) With which one of the following solvents is DMSO not miscible: water, acetone,...
-
Thiols of low molecular mass are known for their extremely foul odors. In fact, the following two thiols are the active components in the scent of the skunk. Give the IUPAC substitutive names for...
-
Kathy Runde, age 66 and retired, receives a $20,000 partial distribution from her 401(k) plan. The plan does not pay out an annuity. Immediately before the distribution, her account balance is...
-
You have been employed as a systems analyst in the information systems organization of a medium-sized consumer goods manufacturer for three years. You are quite surprised when your manager offers you...
-
For your initial post, address the following: First, introduce yourself to the class by sharing a bit about yourself, such as your preferred name or pronouns, where you are from, what your major is,...
-
Question 8 : Consider the technology of Solar Panels. Which stage of the technology life cycle S curve is this technology in. Justify why ? Question 9 : The standard Product Life Cycle has 5 stages...
-
At Benihana restaurant a man wrenched his neck while ducking a piece of flying shrimp, requiring treatment by several doctors. By that summer, doctors determined surgery was necessary to treat...
-
You have just come into an inheritance of $25,000 from a distant relative, and you want to invest it for the long term. Provide an investment portfolio that includes five different stocks. Report the...
-
List the parts of the limbic system, and explain its functions.
-
Express mass density in kg/m3 and weight density in lb/ft3. 1. Find the mass density of a chunk of rock of mass 215 g that displaces a volume of 75.0 cm3 of water. 2. A block of wood is 55.9 in. x...
-
Some NMR spectrometers operate at 4 x 108Hz (400MHz), what is the energy of this radiation?
-
Explain which of these bonds has the absorption for its stretching vibration at higher wave number: (a) C H or C D (b) C = C or C C (c) C C1 or C I
-
Indicate the positions of the absorption bands and any other noteworthy features in the hydrogen region of the IR spectra of thesecompounds: CH3 ) C,CH b) NH2 d) CH2=CHCH,CH,OH f) CH,CH,CH,CH e)...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


