Thiols of low molecular mass are known for their extremely foul odors. In fact, the following two
Question:
Thiols of low molecular mass are known for their extremely foul odors. In fact, the following two thiols are the active components in the scent of the skunk. Give the IUPAC substitutive names for these compounds.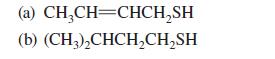
Transcribed Image Text:
(a) CH₂CH=CHCH₂SH (b) (CH3)₂CHCH₂CH₂SH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
ANSWER a The IUPAC substitut...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The end-of-year parties at Yearling, Inc., are known for their extravagance. Management provides the best food and entertainment to thank the employees for their hard work. During the planning for...
-
The end-of-year parties at Golden Beach, Inc., are known for their extravagance. Management provides the best food and entertainment to thank the employees for their hard work. During the planning...
-
Case Study: The Container Store: An Employee-Centric Retailer Introduction : It seems odd that a store would choose to specialize in selling containers and storage units, let alone make a profit from...
-
3. How do the marginal costs of pollution reduction and the marginal costs of pollution damage change as pollution levels increase? The marginal costs of pollution reduction remain constant and the...
-
In the Centrifugal Casting case, what international event made the LOC invaluable to the seller?
-
A rectangular beam with semicircular notches, as shown in part (b) of the figure, has dimension h = 120mm and h1 = 100mm. The maximum allowable bending stress in the plastic beam is max = 6MPa, and...
-
Describe the benefits and risks of global sourcing. 1239
-
Allen Abbott has a wide-curving, uphill driveway leading to his garage. When there is a heavy snow, Allen hires a local carpenter, who shovels snow on the side in the winter, to shovel his driveway....
-
The regular working days in a factory are Monday to Friday. Pete and Bec who are being paid 25 and 27 per hour, respectively, worked on Jobs 31 and 32 for the period August 4 to 10. The weekly time...
-
Give an IUPAC name for each of the following compounds, which may have been isolated from the shoes of a tennis player. Ignore stereochemistry in (a). (a) (b) HO
-
Give the IUPAC substitutive name for each of the following compounds, which have been used as general anesthetics. (a) Br I H-C-CF3 I Cl halothane (b) ClCH-CF-OCH3 methoxyflurane
-
The following data represent the selling price (in thousands of dollars) of oceanfront condominiums in Daytona Beach Shores, Florida. (a) Draw a boxplot of the data. Explain why a t-interval should...
-
As the human resource manager, how would you evaluate the training needs of your staff? How can you ensure that the training you would provide is effective? What data might be used to make your...
-
MARYLAND CORPORATION manufactures three liquid products - Alpha, Beta and Gamma using a joint process with direct materials, direct labor and overhead totaling $560,000 per batch. In addition, the...
-
Three common organizational structures. Mention one organization for each organizational structure which is following a specific organizational structure. Also, provide support to your answer by...
-
You are a retail manager at Kitchen Nightmare, a relatively new store at the mall that sells mostly items for kitchens, like forks, oven mitts, etc.. You have been open since the fall of 2021 and...
-
Examine the extent to which the Department of Veteran Affairs has established any processes or procedures to ensure knowledge retention of departing employees. Why is it important to manage the...
-
Explain the conversion of short-term to tong-term memory.
-
The first national bank pays a 4% interest rate compound continuously. The effective annual rate paid by the bank is __________. a. 4.16% b. 4.20% c. 4.08% d. 4.12%
-
Explain why the presence of a triple bond is much easier to detect in the IR spectrum of 1-hexyne than it is in the spectrum of3-hexyne. 80 60 O The sp-hybridized CH absorption bands: from 3000-2850...
-
The exhaust from a poorly maintained automobile may contain a wide variety of different hydrocarbon pollutants. Why is the 3000 to 2900 cm1 region a good place to monitor the amount of these...
-
Predict the positions of the absorption bands in the IR spectra for the carbonyl groups of thesecompounds. HO. b) CH,CH-CHCH (p
-
Discuss American History
-
Your firm has developed a new lithium ion battery polymer that could enhance the performance of lithion ion batteries. These batteries have applications in many markets including cellphones, laptops,...
-
Need help analyzing statistical data 1. ANOVA) True or false: If we assume a 95% confidence level, there is a significant difference in performance generally across all groups. 2. (t-test) True or...

Study smarter with the SolutionInn App


