To which classes of sugars do the following monosaccharides belong? Which are d and which are L?
Question:
To which classes of sugars do the following monosaccharides belong? Which are d and which are L?
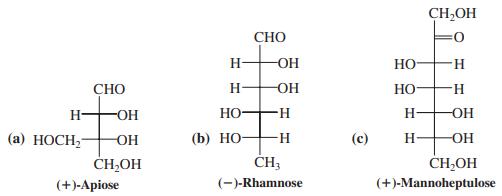
Transcribed Image Text:
CH,OH СНО H- OH НО H. СНО H- OH Но- H- H- -OH HO H OH (а) НОСН OH (b) Но— H- OH- CH,OH CH3 CH,OH (+)-Аpiose (-)-Rhamnose (+)-Mannoheptulose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
To find out D or L we have to look at the lowest most ...View the full answer

Answered By

Saptarshi Paul
Education--
#Completed my Major in Chemistry in 2018
#Currently pursuing my Master's in Chemistry from IIT Kanpur
Tutoring Experience--
#Taught undergraduate level Chemistry to two students for a period of 6 months.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Classify the following monosaccharides. (Examples: D-aldohexose, L-ketotetrose.) (a) (+)-glucose (b) (-)-arabinose (c) L-fructose (d) (e) (f) (g) CHO HO HO OH HO CH,OH +)-gulose CH,OH HOH CH,OH...
-
This problem is meant to encourage you to think as a team about how you might establish the structure of a simple disaccharide, with some additional information at your disposal. Consider d-lactose...
-
Which configuration (R or S) does the bottom asymmetric carbon have for the D series of sugars? Which configuration for the L series?
-
1. Create your Dream Board (all the things you want to buy or establish in the future). Specify the amount of each item. 2. Search the salary of your prospective careers 5 years from now. 3. With the...
-
In testing for co integration between gfr and pe in Example 18.5, add t2 to equation (18.32) to obtain the OLS residuals. Include one lag in the augmented DF test. The 5% critical value for the test...
-
The early atmosphere of the earth probably consisted of carbon dioxide, water vapor, and nitrogen, with little free oxygen. What is believed to be the source of oxygen in the present-day atmosphere?...
-
10. (a) Observe that Ixy - abl = Ixy - xb + xb - abl and Ixl < lal + E.
-
Pet Designs makes various accessories for pets. Their trademark product, PetBed, is perceived to be high quality but not extravagant, and is sold in a variety of pet stores. Wanda Foster, marketing...
-
Pension plan assets were $260 million at the beginning of the year. The return on plan assets was 5%. At the end of the year, retiree benefits paid by the trustee were $11 million and cash invested...
-
An investment requires $16,000 today, and produces the first cash flow of $800 in two years (year 2). Cash flow is expected to grow at 2% per year after year 2. a) What is the NPV of this investment...
-
The designations d and l as applied to sugars refer to the configuration of the highest-numbered stereocenter. If the configuration of the highest numbered stereocenter of d-ribose (Figure 24-1) is...
-
Draw open-chain (Fischer-projection) structures for L-(+)-ribose and L-(-)-glucose (see Exercise 24-2). What are their systematic names? Exercise 24-2 Give a systematic name for (a) D-(-)-ribose and...
-
Discuss the accounting treatment and disclosure of translation adjustments. When does the translation adjustment account have a debit balance? When does it have a credit balance?
-
Q7 a) Two forces equal to 2P and P act on a particle. If the first be doubled and second is increased by 12N, the direction of resultant remains unaltered. Find the value of P (5)
-
On July 1, 2021, P Company borrowed P160,000 to purchase 80 percent of the outstanding common stock of S Company. This loan, carrying a 10 percent annual rate, is payable in 8 annual installments...
-
Case Analysis Strategic leaders, being at the highest level of an organization, are responsible for charting its path to success. They visualize an ideal picture of their enterprise in a futuristic...
-
3 Refrigerant-134a enters a adiabatic compressor at 100 kPa and -24C with a flow rate of 1.300 m/min and leaves at 800 kPa and 60C. Determine the mass flow rate of R-134a and the power input to the...
-
The following trial balance of Bramble Traveler Corporation does not balance. Bramble Traveler Corporation Trial Balance April 30, 2025 Debit Credit Cash $6,221 Accounts Receivable 5,350 Supplies...
-
Create a JavaFX application that implements a short survey. The first question should ask the user for his or her favorite color and present the choices red, orange, blue, and green in radio buttons....
-
What did Lennox gain by integrating their WMS, TMS, and labor management systems?
-
Identify the acids and bases in the followingreactions: CH2 (a) CH3 + Ht TICIA () + TICI, CH "CH (c) + NaH +. Na+ 2 (d) +
-
Which of the following pairs represent resonance structures? (a) CH3C=N-O: and CH3C=N-o: (b) :0: :C C0: and (d) (c) : and :CH2-N CH2=N NH3 and NH2
-
Draw as many resonance structures as you can for the following species. Adding appropriate formal charger to each: (a) Nitromethane, (b) Ozone, (c) Diazomethane, :0: +// H3C-N part a :0: part b...
-
Rocky Mountain Chocolate Factory (RMCF) founder and president Frank Crail employs 220 people in 361 outlets in the United States, Canada, United Arab Emirates, Japan, South Korea and Saudi Arabia. If...
-
Lakeland Inc. manufactured 2,500 units during the month of March. They incurred direct materials cost of $58,000 and overhead costs of $40,000. If their per-unit prime cost was $32.00 per unit, how...
-
The market price of a semi-annual pay bond is $979.86. It has 21.00 years to maturity and a yield to maturity of 7.34%. What is the coupon rate? Submit Answer format: Percentage Round to: 0 decimal...

Study smarter with the SolutionInn App


