Typical conditions for nitrosation are illustrated in the following equation. Propose a detailed mechanism for this reaction.
Question:
Typical conditions for nitrosation are illustrated in the following equation. Propose a detailed mechanism for this reaction.
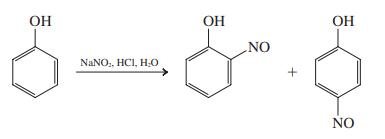
Transcribed Image Text:
OH OH ОН NO NANO,, HCI, H0 NO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Mechanism OH No nitrexsation Is th...View the full answer

Answered By

Afsar Ahmed
Hello. I am Afsar. I have been graduated in Chemistry in the year 2018. I am also a student and pursuing masters (M.Sc) & hopefully will be completing the degree in the 2020. I will be happy to solve problems in the concerned subject that I have been studying for last few years .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Propose a mechanism for each reaction. (a) (b) Ht H3C CH3 OH H SOA Ph Ph Ph Ph
-
Propose a mechanism for each reaction. (a) (b) (c) (d) OH H2SO heat OCH + CH3OH H20 CH2OH H2So4 heat CH OCH2CH CH CH,CH2OH (a minor product)
-
(a) Mitomycin is a clinically used antitumor antibiotic that acts by disrupting DNA synthesis through covalent bondforming reactions with deoxyguanosine in DNA. Maria Tomasz (Hunter College) and...
-
Craps is a dice game in which two fair dice are cast. If the roller shoots a 7 or 11 on the rst roll, he or she wins. If the roller shoots a 2, 3, or 12 on the rst roll, he or she loses. (a) Compute...
-
Wells filed a lawsuit against her employer Clackamas Gastorenterology Associates, P.C. alleging that the medical clinic violated the Americans with Disabilities Act of 1990 (ADA or Act) when it...
-
The science of encryption is known as which of the following? a. Cryptanalysis b. Steganography c. Cryptology d Algorithm
-
1 7 Explain the difference between Herzbergs hygiene and motivating factors.
-
Branson paid $465,000 cash for all of the outstanding common stock of Wolfpack, Inc., on January 1, 2011. On that date, the subsidiary had a book value of $340,000 ( common stock of $200,000 and...
-
Cash Discounts You place an order for 380 units of inventory at a unit price of $130. The supplier offers terms of 1/10, net 30. a. How long do you have to pay before the account is overdue? If you...
-
A recent study by researchers at North Carolina State University found thousands of errors in 12 of the most widely used high school science texts. For example, the Statue of Liberty is left-handed;...
-
The nitroso group, NO, as a substituent on a benzene ring acts as an ortho, para directing group but is deactivating. Use the Lewis structure of the nitroso group and its inductive and resonance...
-
Polystyrene (polyethenylbenzene) is a familiar polymer used in the manufacture of foam cups and packing beads. One could, in principle, synthesize polystyrene by cationic polymerization with acid....
-
How are service department costs charged to responsibility centers?
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Briefly explain what spatial autocorrelation means and what method can be used to measure it
-
Discuss the theoretical implications of adopting biodegradable materials in civil engineering for reducing environmental impact and enhancing sustainability.
-
Shake Shack, Inc. began operations in 2014, and it incorporated in Delaware in Year 1. In February, 2015 it went public with its initial public offering of common stock. By December 31, 2017 it was...
-
After looking at the resources, explain what a spirit image is. Why might looking at a god and/or a human in terms of their spirit be helpful if you want to eliminate some of the divisions between...
-
A first -order decomposition reaction is observed to have the following rate constants at the indicated temperatures. Estimate the activation energy. k/(10-3 s-1) 2.46 45.1 576 0/C 20.0 40.0
-
Sucrose is readily hydrolyzed to glucose and fructose in acidic solution. The hydrolysis is often monitored by measuring the angle of rotation of plane polarized light passing through the solution....
-
Show that the following mechanism can account for the rate law of the reaction in Problem 22.11: What further tests could you apply to verify this mechanism? HCl + HCl K, HCI + CH,CH=CH, complex...
-
At the end of April K Marx company received their bank statement that showed an ending balance of $5,875. K Marx company's balance in their general ledger cash account was $6,000. Based on the...
-
A firm has a WACC of 8.86% and is deciding between two mutually exclusive projects. Project A has an initial investment of $62.59. The additional cash flows for project A are: year 1 = $18.74, year 2...
-
[ The following information applies to the questions displayed below. ] Tunstall, Incorporated, a small service company, keeps its records without the help of an accountant. After much effort, an...

Study smarter with the SolutionInn App


