Question: Upon completing a synthetic procedure, every chemist is faced with the job of cleaning glassware. Because the compounds present may be dangerous in some way
Upon completing a synthetic procedure, every chemist is faced with the job of cleaning glassware. Because the compounds present may be dangerous in some way or have unpleasant properties, a little serious chemical thinking is often beneficial before “doing the dishes.” Suppose that you have just completed a synthesis of hexanoyl chloride, perhaps to carry out the reaction in Problem 34(b); first, however, you must clean the glassware contaminated with this acyl halide. Both hexanoyl chloride and hexanoic acid have terrible odors.
(a) Would cleansing the glassware with soap and water be a good idea? Explain.
(b) Suggest a more pleasant alternative, based on the chemistry of acyl halides and the physical properties (particularly the odors) of the various carboxylic acid derivatives.
Data From problem 34 (b)
(b)
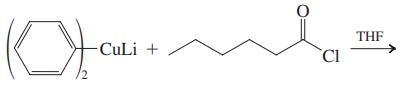
THF CuLi + CI
Step by Step Solution
3.48 Rating (165 Votes )
There are 3 Steps involved in it
Answer a No absolutely not Using soap water would be a terrible idea as hexanol chloride ... View full answer

Get step-by-step solutions from verified subject matter experts


