Use the following partial IR- and mass-spectral data to identify one of the structures among the selection
Question:
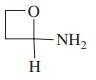
Use the following partial IR- and mass-spectral data to identify one of the structures among the selection given. IR spectrum: 3300 and 1690 cm-1; mass spectrum: m/z = 73 (parent ion).
(a)
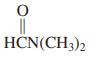
(b)
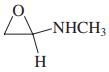
(c)
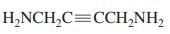
(d)
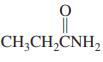
(e)
Transcribed Image Text:
HCN(CH3)2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 28% (14 reviews)
Given that mass spectrum mt 73 IR spet...View the full answer

Answered By

Appala naidu karri
Having taught in special education in two local schools for many years meant that I had contact with a lot of parents of special needs students. I never had to advertise — word of mouth was how most folks knew of me. At one point I did have a website, but didn't utilize it much. I stayed very busy, especially in the summers, and always had a full schedule. I typically met with each student's teacher in order to get an idea of what the focus of my instruction/remediation should be. Becoming familiar with the student's learning style(s) was also very helpful. Often parents would share records and test results with me. After each tutoring session, I documented the student’s progress and gave parents written updates, as well as phone calls or emails as neede
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Identify each of the following compounds from their spectra. (a) Compound A: molecular mass 113; gives a positive hydroxamate test; IR 2237, 1733, 1200 cm-1; proton NMR: 1.33 (3H, t, J = 7 Hz), ...
-
The mass spectrum of unknown compound A shows a molecular ion at m/z 116 and prominent peaks at m/z 87 and m/z 101. Its UV spectrum shows no maximum above 200 nm. The IR and NMR spectra of A follow....
-
Use the following NMR- and mass-spectral data to identify the structures of two unknown compounds, A and B. A: 1 H NMR: = 0.92 (t, J = 6 Hz, 3 H), 1.32 (broad s, 12 H), 2.28 (broad s, 2 H), and 2.69...
-
The advantages of the computerized conversion process model " What is the EOQ model? (For self-study and research) What is JIT? (Self study and research) A firm expects to sell 2000 units of its...
-
What are the major types of transactions or activities that result in supply of foreign currency in the spot foreign exchange market?
-
Assume that the entity is a charity that charges its members monthly dues totaling $100,000 per year (in both Year 1 and Year 2). However, the members get nothing for their dues. The organization has...
-
Kawasaki Corporation borrowed $50,000 on November 1, 2004, by signing a $51,125, 3-month, zero-interest-bearing note. Prepare Kawasakis November 1, 2004, entry; the December 31, 2004, annual...
-
Required Tasks: 1. Prepare a descriptive analysis of the data using charts, graphs, and numerical measures. 2. Construct and interpret a 95% confidence interval estimate for the mean weight for male...
-
3. Praeger Company began operations on January 1 and produces a single product that sells for $10,00 per unit. Standard capacity is 100,000 units per year. During the year, 100,000 units were...
-
The Katash Company is a leader in the poultry market. It produces, sells and markets fresh and ice packed commodity chicken and frozen products known for their value and healthful qualities. Katash's...
-
Which of the following formulas best represents diazomethane? (a) (b) (c) (d) (e) CH,=N=N:
-
Give the expected major product(s) of each of the following reactions. (a) (b) CH,CH3 CI.(1 equivalent), hv
-
Air is flowing steadily through a horizontal tube at a constant temperature of $32^{\circ} \mathrm{C}$ and a mass flow rate of $1 \mathrm{~kg} / \mathrm{s}$. At one point upstream where the tube...
-
The 150 m long beam is submitted to a distributed load w(x) = (0.05 x 2) + 10 N/m. 50 50 w(x) 100 150 What is the moment about the point O in kN.m created by the distributed load? O-25.7 kN.m O-249...
-
X Calculate the reaction rate at various conversions, as shown below: FAO -TA -TA 0.2 0.8 Considering that for a PFR: dx V = FAO What is the conversion reached after the 50 m of this PFR?
-
For decades, leaders have talked about flexible working options, yet only few companies were consistently using these practices prior to the global health crisis of 2020. In March 2020, organizations...
-
Manatee Corp. has developed standard costs based on a predicted operating level of 352,000 units of production, which is 80% of capacity. Variable overhead is $281,600 at this level of activity, or...
-
When leaders are facing a crisis or an opportunity, generally, they tend to fall back on the leadership style that has worked for them in the past. Discuss with examples the options that would help...
-
Write a program called FightSong that produces this output. Use at least two static methods to show structure and eliminate redundancy in your solution. Go, team, go! You can do it. Go, team, go! You...
-
Several months have passed and the Managing Partner approved and properly filed the Complaint and properly submitted the Request for Production of Documents that you drafted. In fact, it has been 75...
-
Explain which of the five products shown in is formed when 1-ethylcycpentene reacts with BH3 in THF, followed by treatment with NaOH andH2O2.
-
Explain which of the three products shown is formed when trans-2-butene reacts with CH2I2 andZn(Cu).
-
Explain which of the three products shown is formed when cis-2-butene reacts with OsO4 and t-BuOOH
-
a dutch auction is the same as an
-
Part 1 . The risk - free rate of return is 3 . 5 percent, the inflation rate is 2 . 9 percent, and the market return ( S&P 5 0 0 ) is 1 0 . 5 percent. What is the expected rate of return on a stock...
-
The Birch Corp. has the following items in its capital structure at 3 1 December 2 0 x 7 , the end of the fiscal year: 0 . Options to purchase 5 1 0 , 0 0 0 common shores were outstanding for the...

Study smarter with the SolutionInn App


