Question: Using the methods described in this chapter, design a multistep synthesis of each of the following molecules, making use of the indicated building blocks as
Using the methods described in this chapter, design a multistep synthesis of each of the following molecules, making use of the indicated building blocks as the sources of all the carbon atoms in your final product.
(a)
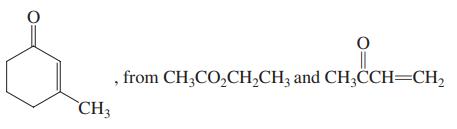
(b)
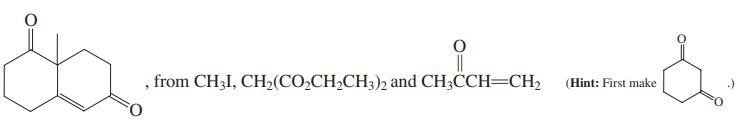
(c)
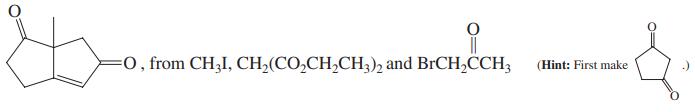
, from CH;CO,CH,CH; and CH3CCH=CH, CH3
Step by Step Solution
3.53 Rating (167 Votes )
There are 3 Steps involved in it
Answers are given in the figures AC Ans for a Figure A Ans for b Figure B A... View full answer

Get step-by-step solutions from verified subject matter experts


