When 2-methylcyclopentanone is treated with the bulky base triphenylmethyllithium under the two sets of conditions shown, the
Question:
When 2-methylcyclopentanone is treated with the bulky base triphenylmethyllithium under the two sets of conditions shown, the two possible enolates are generated in differing ratios. Why is this so?
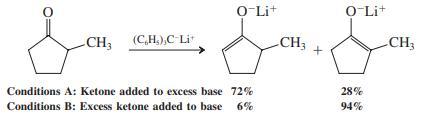
To tackle this problem, you have to invoke the principles of kinetic versus thermodynamic control; that is, which enolate is formed faster and which one is more stable? Divide your team so that one group considers conditions A and the other conditions B. Use curved arrows to show the fl ow of electrons leading to each enolate. Then assess whether your set of conditions is subject to enolate equilibration (thermodynamic control) or not (kinetic control). Reconvene to discuss these issues and draw a qualitative potential-energy diagram depicting the progress of deprotonation at the two α sites.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





