Which of the following reactions is an electrophilic aromatic substitution? Se, 300 Cl, hv (a) C,H12 (b)
Question:
Which of the following reactions is an electrophilic aromatic substitution?
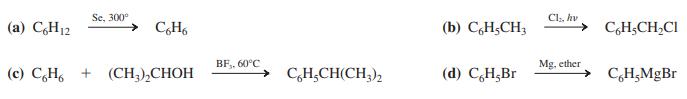
Transcribed Image Text:
Se, 300° Cl, hv (a) C,H12 (b) C,H;CH3 CH;CH,CI BF,, 60°C Mg. ether (c) C,H, + (CH,),CHOH C,H,CH(CH;), (d) CH,Br C,H;MgBr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
C6H6 CH32 CHOH ...View the full answer

Answered By

Seenaiah Gangipaka
I am post graguate(MSc-Organic Chemistry) and B.Ed(Physical Science).I have 11 years of good experience in teaching and professional.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Which compound will undergo an electrophilic aromatic substitution reaction more rapidly, benzene or hexadeuteriobenzene? or D- H.
-
For each of the groups of substituted benzenes, indicate a. The one that would be the most reactive in an electrophilic aromatic substitution reaction b. The one that would be the least reactive in...
-
Sulfonation, unlike most other electrophilic aromatic substitution reactions, is reversible. Benzenesulfonic acid (structure in Eq. 16.12, p. 755) can be converted into benzene and H2SO4 with hot...
-
The probability that fewer than 35 people support theprivatization of Social Security A discrete random variable is given. Assume the probability of the random variable will be approximated using the...
-
The issue in this bankruptcy proceeding is whether a debtor who was under a personal service contract is entitled to reject the contract after filing bankruptcy. In this case, the debtor, a movie and...
-
I. Comprehend that symmetric encryption is also known as public-key encryption. II. Explain that symmetric encryption uses a single key to encrypt and decrypt, but asymmetric encryption uses two...
-
1 4 How does Maslows theory of human needs relate to the ideas of Frederick Taylor?
-
The chief accountant for Dollywood Corporation provides you with the following list of accounts receivable written off in the current year. Dollywood Corporation follows the policy of debiting Bad...
-
help with the journal entries! Chart of Accounts \begin{tabular}{|c|c|} \hline \multicolumn{2}{|c|}{ Current Assets } \\ \hline 10000 & Cash \\ \hline 11000 & Accounts Receivable \\ \hline 12000 &...
-
Under the Affordable Care Act, what are the minimum essential coverage tests that the insurance provided by the employer must satisfy?
-
Polystyrene (polyethenylbenzene) is a familiar polymer used in the manufacture of foam cups and packing beads. One could, in principle, synthesize polystyrene by cationic polymerization with acid....
-
The intermediate cation A in the sequence C 6 H 6 + E + A C 6 H 5 E + H + is best shown as H H E H E () (b) () (d) `E
-
Debating if employees have a duty to blow the whistle on corporate misconduct, or if employees should always be loyal to their employer.
-
When to use a tuple vs list vs dictionary in Python? Explain some benefits of Python
-
What is Lambda Functions in Python? How do I modify a string in python?
-
1. How will you check if a class is a child of another class? 2. What is init method in python?
-
1. What are lists and tuples? What is the key difference between the two? 2. What is Scope in Python?
-
1. What is an Interpreted language? 2. What is a dynamically typed language?
-
The following three companies issued the following bonds: 1. Lot, Inc. issued $100,000 of 8 percent, five-year bonds at 102 on January 1, Year 1. Interest is payable annually on December 31. 2. Max,...
-
The Dow Jones Industrial Average reached a high of $ 7801.63 on December 29, 1997. Recall from Example 18.4 that it reached a high of $ 1003 on November 14, 1972. The Consumer Price Index for...
-
The equilibrium A ( B is first -order in both directions. Derive an expression for the concentration of A as a function of time when the initial molar concentrations of A and Bare [A]0 and [B]0. What...
-
Derive the integrated form of a third-order rate law v = k[A f [B] in which the stoichiometry is 2 A + B ( P and the reactants are initially present in (a) Their stoichiometric proportions, (b) With...
-
Show that the ratio t1/2/t3/4 where 1112 is the half-life and 13/4is the time for the concentration of A to decrease to t of its initial value (implying that t3/4 < t1/2) can be written as a function...
-
in a bank reconciliation which of the following items are added to the end of the month ledger cash account balance
-
The outstanding bonds of panda express are priced at 1100 and mature in 3 years. These bonds have an 8 percent coupon and pay interest annually. The face value is 1000. The firms tax rate is 40...
-
Steeler Towel Company estimates its overhead to be $250,000. It expects to have 100,000 direct labor hours costing $2,500,000 in labor and utilizing 12,500 machine hours. Calculate the predetermined...

Study smarter with the SolutionInn App


