Which of the three compounds below would be the most reactive toward hydrolysis with aqueous base? (a)
Question:
Which of the three compounds below would be the most reactive toward hydrolysis with aqueous base?
(a)
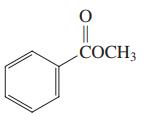
(b)
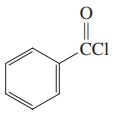
(c)
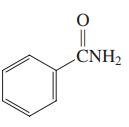
Transcribed Image Text:
СОСH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
correct option is b Explanation These are all acid derivatives The reactivity of a...View the full answer

Answered By

Showket Ahmad khoja
I have Masters degree in chemistry and I am topper of my batch. I have also Qualified J&K SET . I am also winner of national Chemistry quiz. I have 5 years of experience in teaching Chemistry to 11th, 12th , NEET, JEE , +2 and +3 levels. I am both offline as well as online tutor. I am currently teaching Chemistry at CM'S Model Boys Higher secondary school Kupwara j&k India
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Which of the three primary composite geometries is most likely to possess isotropic properties?
-
Which compound is more reactive toward electrophilic substitution (for example, nitration)? OCH or b. CH,CH3 a. ETor
-
Which of the three basic philosophies of social responsibility would you find most appealing as the chief executive of a large corporation? Explain.
-
Line A: y = 3 0.6x Line B: y = 4 x a. Graph the linear equations and data points. b. Construct tables for x, y, Ëy, e, and e 2 similar to Table 4.4 on page 151. c. Determine which line fits the...
-
Looking at the last year of the data, compare the average openness of the 20 largest countries (measured in terms of population) and the 20 smallest countries. Which is more open?
-
What is a vector? A vector function? A vector field? A scalar? A scalar function? A scalar field? Give examples.
-
(Current versus Noncurrent Classification) DAnnunzio Corporation includes the following items in its liabilities at December 31, 2004. 1. Notes payable, $25,000,000, due June 30, 2005. 2. Deposits...
-
A process fluid having a specific heat of 3500 J/kg K and flowing at 2 kg/s is to be cooled from 80C to 50C with chilled water, which is supplied at a temperature of 15C and a flow rate of 2.5 kg/so...
-
Which of the following statements is true regarding an intra-entity transfer of land? Multiple Choice a. A loss is always recognized but a gain is deferred in a consolidated income statement. b. A...
-
The Trial Balance section of the worksheet for 21st Century Fashions for the period ended December 31, 2016, appears on the next page. Adjustments data are also given. ADJUSTMENTS a. Supplies used,...
-
The best description for compound A (see margin) is (a) An amide; (b) A lactam; (c) An ether; (d) A lactone. H2C-C H2C-O A
-
Give the products of reaction of methyl pentanoate with each of the following reagents under the conditions shown. (a) NaOH, H 2 O, heat; then H + , H 2 O (b) (CH 3 ) 2 CHCH 2 CH 2 OH (excess) , H +...
-
A diffuse, gray radiation shield of 60-mm diameter and emissivities of ? 2, i = 0.01 and ? 2, o = 0.1 on the inner and outer surfaces, respectively, is concentric with a long tube transporting a hot...
-
A company is issuing $340,000 worth of 4-year bonds on October 8, 2023, bearing an interest rate of 2%, payable annually. Assume that the current market rate of interest is 3%. a) Will the bonds be...
-
Use the Comprehensive Annual Financial Report for the Village of Arlington Heights (please look up this content) for the year ended December 31, 2018, to answer questions 8-20. All questions are on...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Two wires lie perpendicular to the plane of the screen and carry equal magnitudes of electric current in the directions shown. Point P is equidistant from the two wires. The distance between each of...
-
Your firm has recently been appointed as auditors of Kentronics Ltd , a large company which markets sophisticated electronic equipment for heavy industry as well as the mining equipment industry. The...
-
Extend your F# code from Exercise C 16.16 to include list removal and search routines. After finding and reading appropriate documentation, package these routines in a library that can be called in a...
-
When the concentration of a strong acid is not substantially higher than 1.0 10-7 M, the ionization of water must be taken into account in the calculation of the solution's pH. (a) Derive an...
-
Show the products of these elimination reactions and indicate which ismajor: OTs . b) CH,OH + CH,0 + OH ELOH CI E:OH + CH,CH,O c)
-
The reaction of 2-bromobutane with ethoxide ion in ethanol gives 81% of a mixture of (Z)- and (E)-2-butene. Explain which stereo isomer you expect to predominate in this mixture.
-
Show the products of these reaction and indicate which ismajor: N(CH3)3 N(CH3)3 a) b) d) c) CH3 .
-
Investment-grade bonds have no default risk. True O False
-
Madrid Company plans to issue 7% bonds on January 1, 2017, with a par value of $5,100,000. The company sells $4,590,000 of the bonds at par on January 1, 2017. The remaining $510,000 sells at par on...
-
Year Cash flow 0 - 7 5 0 , 0 0 0 , 0 0 0 1 1 5 0 , 0 0 0 , 0 0 0 2 1 8 0 , 0 0 0 , 0 0 0 3 1 9 5 , 0 0 0 , 0 0 0 4 2 3 5 , 0 0 0 , 0 0 0 5 2 2 0 , 0 0 0 , 0 0 0 6 1 8 5 , 0 0 0 , 0 0 0 7 1 6 5 , 0 0...

Study smarter with the SolutionInn App


