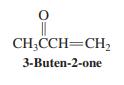
Would you expect addition of HCl to the double bond of 3-buten-2-one (shown in the margin) to
Question:
Would you expect addition of HCl to the double bond of 3-buten-2-one (shown in the margin) to follow Markovnikov’s rule? Explain your answer by a mechanistic argument.
Transcribed Image Text:
CH;CCH=CH, 3-Buten-2-one
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Q Would you expect addition of HCl to the double bond ...View the full answer

Answered By

Seenaiah Gangipaka
I am post graguate(MSc-Organic Chemistry) and B.Ed(Physical Science).I have 11 years of good experience in teaching and professional.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
What two products are possible from the addition of HCl to 2-octene? Would you expect the reaction to be regiospecific?
-
What product(s) would you expect to obtain from reaction of 1, 3-cyclo-hexadiene with each of the following? (a) 1 mol Br2 in CH2C12 (b) O3 followed by Zn (c) 1 mol HCI in ether (d) 1 mol DCl in...
-
(a) How many stereoisomers are possible for 9, 10-dibromohexadecanoic acid? (b) The addition of bromine to palmitoleic acid yields primarily one set of enantiomers, ()-threo-9, 10-dibromohexadecanoic...
-
If a check correctly written and paid by the bank for $ 648 is incorrectly recorded on the company's books for $ 684 , the appropriate treatment on the bank reconciliation would be to THE ANSWER IS...
-
Matthew works as a paralegal for the prosecutor's office. While organizing a file in preparation for trial he comes across the name and telephone number of a witness who identified the perpetrator of...
-
Is the given function even or odd or neither even nor odd? Find its Fourier series. Show details of your work.
-
1 Why do you think each takes the form they do?
-
1. Evaluate the concept of administrative liaison officer as a strategy for achieving integration. Is this an example of the mutual adjustment strategy? 2. How will the officers achieve integration...
-
Ivanhoe Company had these transactions during the current period. June 1 2 Issued 8 6 , 5 0 0 shares of $ 1 par value common stock for cash of $ 3 2 4 , 3 7 5 . July 1 1 Issued 3 , 1 0 0 shares of $...
-
Inspire Inc. has a 49% ownership stake in Baldwin Industries. Crown Company has a 24% stake in Baldwin Industries. Inspire Inc. has been offered $19.6 Million for 80% of its stake. If Crown Company...
-
Propose syntheses of the following compounds by using Michael additions followed by aldol condensations (i.e., Robinson annulation). Each of the compounds shown has been instrumental in one or more...
-
Using the following information, propose structures for each of these compounds. (a) C5H10O, NMR spectrum A, UV max (e) 5 280(18) nm; (b) C5H8O, NMR spectrum B, UV lmax(e) 5 220(13,200), 310(40) nm;...
-
Dividing net income by total assets produces a ratio known as _____ _____ _____, which indicates how effectively the organization is using its assets to earn additional profits.
-
Max 1 page allowed] Consider a DRAM chip of capacity 256 KB and each memory location contains 8 bits. The memory chip is organized in matrix form with equal number of rows and column for each memory...
-
find the dimensions of a notman window of perimeter 3 9 ft that will admit the greatest possible amount of light. Round answer to two decimal places
-
Consider the expression timing is everything in relation to the building of the TOMS brand. Besides the influence of recovering economic conditions and the increased affluence of potential customers,...
-
What is corporate strategy and why is it important? Choose a company with which you are familiar, and evaluate its corporate strategy, especially in regards to financial strategies. What are some...
-
Assignment Tasks: Review the following situations and for each pay period determine the employee's net pay by calculating what earnings & benefits are subject to Income Tax, Canada / Quebec Pension...
-
As of June 30, Year 2, the bank statement showed an ending balance of $19,500. The unadjusted Cash account balance was $15,200. The following information is available: 1. Deposit in transit, $2,400....
-
What is master production scheduling and how is it done?
-
The transfer coefficient of a certain electrode in contact with M2+and M3+ in aqueous solution at 25C is 0.42. The current density is found to be 17.0 mA cm when the over voltage is 105 may. What is...
-
Determine the exchange current density from the information given in Exercise 25.16b.
-
Determine the effect that increasing the over potential from 0.50 V to 0.60 V has on the current density in the electrolysis of 1.0 M NaOH (aq), which is 1.22 mA cm-2 at 0.50 V and 25C. Take a= 0.50.
-
Calculate the Banks Duration Gap Calculate the Change in the Banks Economic Value of Equity Calculate the Change in the Banks Economic Value of Equity
-
Abner Corporation's bonds mature in 16 years and pay 13 percent interest annually. If you purchase the bonds for $825, what is your yield to maturity?
-
A company reports the following sales-related information. Prepare the net sales portion only of this company's multiple-step income statement

Study smarter with the SolutionInn App


