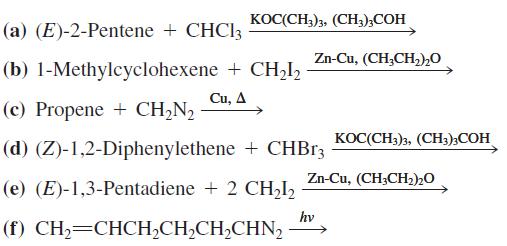
Write the expected products of each of the following reactions. KOC(CH3)3, (CH,);COH (a) (E)-2-Pentene + CHCI3 Zn-Cu,
Question:
Write the expected products of each of the following reactions.
Transcribed Image Text:
KOC(CH3)3, (CH,);COH (a) (E)-2-Pentene + CHCI3 Zn-Cu, (CH,CH,)2O (b) 1-Methylcyclohexene + CH,I, Си, Д (c) Propene + CH,N, KOC(CH3)3, (CH;);COH (d) (Z)-1,2-Diphenylethene + CHB13 Zn-Cu, (CH;CH2)20 (e) (E)-1,3-Pentadiene + 2 CH2I2 hv (f) CH,=CHCH,CH,CH,CHN,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
a We get ccl 2 as a carbene which got inserted into the duoble bond of the alkene b This is ...View the full answer

Answered By

Unik arora
right now i'm in final semester of my masters in chemistry . my strong space is organic and physical chemistry.
till now i don't have any tutoring experience but my main focus is always on build fundamental of subject .so my teaching / studying methodology is based on fundamental . i cleared a bug of easy exams like IIT JAM ,CSIR-NET,GATE ,JEE MAINS
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Suggest a reasonable mechanism for each of the following reactions:
-
Write structural formulas for the major organic products from each of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) O (excess), H SO (cat.) OH 0 H2O, H,so, (cat.) (1) Mg (2) CO2 (3) H2O Br...
-
Write structural formulas for the major organic products from each of the following reactions. (a) (b) (c) (d) (e) CI CH CH.SH NH2 (excess Cl OH H SO, (cat.) HO AlCl3
-
Suggest two reasons why the adjustments proposed by independent auditors more often than not call for reducing recorded earnings.
-
Build a quality management plan for the other two R & S Amusements Services selected projects customer contract management or collection management.
-
The probability that a specific vulnerability within an organization will be the target of an attack is known as which of the following? a. Probability b. Manageability c. Likelihood d. Practicality
-
Was this an inappropriate strategy? LOP4
-
The financial statements of P&G are presented in Appendix 5B or can be accessed at the books companion website, www.wiley.com/college/kieso. Instructions Refer to P&Gs financial statements and the...
-
Problem I Prepare a master budget for McGregor Pharmacy Company for the year ending December 31, 2020 using the following information. Prepare it per quarter. Use the tables provided by the...
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...
-
Propose a mechanism for the peroxide-initiated reaction of CH 3 SH with 1-hexene.
-
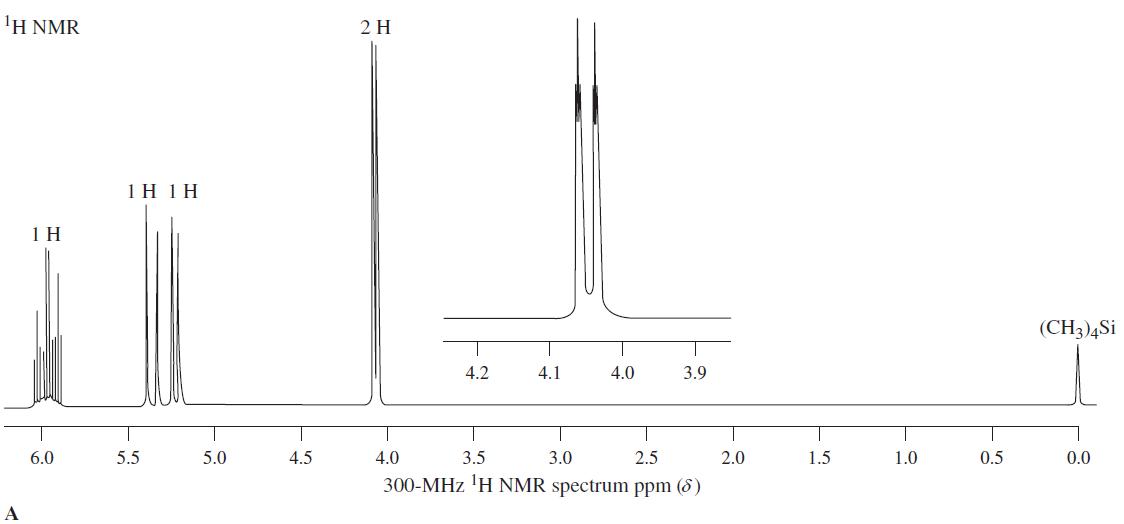
1 H NMR spectrum A corresponds to a molecule with the formula C 3 H 5 Cl. The compound shows significant IR bands at 730 (see Problem 53 of Chapter 11), 930, 980, 1630, and 3090 cm -1 . (a) Deduce...
-
Go to the campus placement office to gather some information on companies that recruit information systems graduates. Try to find any information about the companies approaches to developing systems....
-
Suppose k(x) = f(g(h(x))). Given the table of values below, determine k' (1). g(x) h(x) f'(x) g'(x) h'(x) x f(x) 1 -6 -3 3 6 -6 -6 3 -3 4 1 -7 -2 5 4 -2 7 3 1 -7 -8
-
In a research study women with metastatic stomach cancer responded to the Symptom Distress Scale and the Profile of Mood States. A correlation coefficient was reported: r = 0.5, p = 0.03. How would...
-
Charlotte, a marketing manager, is worried her firm is doing a poor job of managing the movement of finished products to the final consumer. If she is right, the company should work to improve its Mul
-
Sketch a graph of the piecewise defined function. f(x) = Sx if x 0 x+9 if x>0
-
Complete the information for the following subnetting problem. Your answers should be whole numbers without a period. Example: the correct value for the last octet of 172.20.55.210 would be entered...
-
Write a method called perfectNumbers that accepts an integer maximum as its parameter and prints all perfect numbers up to and including that maximum. A perfect number is an integer that is equal to...
-
[a] Two foam blocks, each with a charge of 19 micro coulombs (1 C = 10-6 C), are both held in place 19 cm apart in the east-west direction. A foam ball with a charge 49 C is placed 55 cm north of the...
-
Express the equilibrium constant of a gas-phase reaction A + 3 B ~ 2 C in terms of the equilibrium value of the extent of reaction, ~, given that initially A and B were present in stoichiometric...
-
Show that, if the ionic strength of a solution of the sparingly soluble salt MX and the freely soluble salt NX is dominated by the concentration C of the latter, and if it is valid to use the...
-
To get a sense of the effect of cellular conditions on the ability of ATP to drive biochemical processes, compare the standard Gibbs energy of hydrolysis of ATP to ADP with the reaction Gibbs energy...
-
Required information Required information Problem 7 - 7 ( Static ) Calculate depreciation of property and equipment and amortization of intangible assets ( LO 7 - 4 , 7 - 5 ) [ The following...
-
Process Costing - Weighted Average Method Work In Process 1 0 / 1 - 1 6 , 0 0 0 units Direct Material: 1 0 0 % complete $ 5 4 , 5 6 0 Conversion Cost: 1 0 % complete $ 3 5 , 5 6 0 Balance WIP 1 0 / 1...
-
A merchandising concern paid $1,600,000 for inventory. The market value of the inventory is $1,200,000. The net realizable value of the inventory is $1,300,000. The company found a new supplier for...

Study smarter with the SolutionInn App


