Write the products of each of the following reactions after aqueous work-up. (e) Write the results that
Question:
Write the products of each of the following reactions after aqueous work-up.
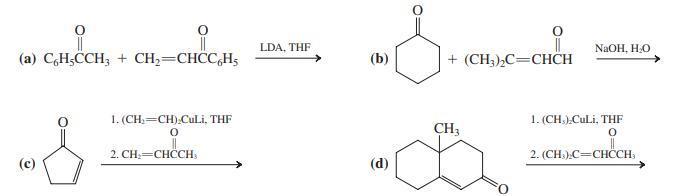
(e) Write the results that you expect from base treatment of the products of reactions (c) and (d).
Transcribed Image Text:
LDA, THE (a) CH;CCH; + CH;=CHCC,H; NaOH, HO + (CH;),C=CHCH 1. (CH,=CH).CULI, THF 1. (CH.).CuLi, THF CH3 2. CН— СНCСH 2. (CH:).C=CHCCH, (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
Results from a wide range of climate model simulations ...View the full answer

Answered By

Dudhat Vaidehi
I tutored mostly elementary school students privately after school and during the summer. We met in their homes or at the public library. I charged an hourly fee, and I provided any necessary materials.
Having taught in special education in two local schools for many years meant that I had contact with a lot of parents of special needs students. I never had to advertise — word of mouth was how most folks knew of me. At one point I did have a website, but didn't utilize it much. I stayed very busy, especially in the summers, and always had a full schedule. I typically met with each student's teacher in order to get an idea of what the focus of my instruction/remediation should be. Becoming familiar with the student's learning style(s) was also very helpful. Often parents would share records and test results with me. After each tutoring session, I documented the student’s progress and gave parents written updates, as well as phone calls or emails as needed.
While my students and I certainly utilized technology and the internet often during our sessions, I never tutored online or for any tutoring company, so am not familiar with the curriculums or methods used in those settings.
Tutoring one on one was very enjoyable and rewarding. My students and I had fun, and grew quite fond of one another. The extra income was a bonus. I had to retire from tutoring due to a physically handicapping disease, and miss my students very much.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the products of each of the following reactions: a. b. c. d. e. f. g. h. i. j. HCI CH2CH3 1. CH3CH2MgBr 1. CH3CH2MgBr excesS CH,CH,COCH 2. H20 ot.cum 1. LiAIH4 NO 2. H20 catalytic Ht + CH...
-
Give the products of each of the following reactions: a. b. c. d. CH,-CH-CH-CH2 + CH3C-C= C-CCH, CH CH CH3 CHa CH3C CH-CH-CCH3
-
Predict the products of each of the following reactions. (a) (b) (c) (d) OH Cl pyridine OH (1) NaH (2) CH2l HBr OH HNOg, H2SO4 H3C
-
The following are selected transactions of Bridgeport Department Store Ltd. for the current year ended December 31. Bridgeport is a private company operating in the province of Manitoba where PST is...
-
Apply the North Carolina assault statute found in Section 4-5 to the following facts. a. Bobby is angry with his supervisor. He takes a gun to work, intending to scare his supervisor. He waves the...
-
Find (a) The Fourier cosine series (b) The Fourier sine series. Sketch f(x) and its two periodic extensions. Show the details. KIN NIA
-
1 Are there any examples of problems in the working relationships between units which may be attributed to their having mechanistic and organic characteristics?
-
1. Are Whole Foods team members likely to experience problems with procedural and/or distributive justice? Explain. 2. Which of the motivational practices are emphasized by Whole Foods in its...
-
Company was destroyed by fire. The current period information that he was able to save was as follows: Beginning Inventory, Jan 1: $34,000 Purchases to date: $118,000 Sales to date: $ 140,000...
-
MSP Corp. repairs watches and clocks. The company is owned and operated by Maria Samuels. As of August 31, 2026, the company had these account balances: Account Balance Cash $46,200 Accounts...
-
Give the expected product(s) of each of the following reactions. 1. LDA, THF 2. BICH,COCH, H 1. LDA, THF 2. CH.CH Br, HMPA (a) CH,CCH,CH,CH3 (b) 0:
-
Write the final products of the following reaction sequences. (a) (b) (c) (d) Write a detailed mechanism for reaction sequence (c). NaOCH, CH,O, O + CH2=CHCCH3
-
Provide an argument for why a variable pricing policy might increase the sales revenue from Apple's Music Store (compared to the flat pricing policy).
-
(1) The Mean Value Theorem states: Let f be continuous over the closed [a, b] and differentiable over the open interval (a, b). Then, there exists at least one point c E (a, b) such that: f(b) - f(a)...
-
Assume you are an Israeli investor; the symbol for the Israeli currency, the shekel, is ILS. You see that stock for Top Image has a bid price of ILS 17 and an ask price of ILS 19 in Israel, a bid...
-
3. Given the continuous beam shown below, which span or spans should be loaded with a uniform distributed load to produce a maximum moment at support B? (5 points) SPAN 1 SPAN 2 SPAN 3 A B D 20 ft...
-
Complete the following writing assignment: Analyze the attached 10_pages. Write_about them, summarize what you read, and connect it to personal experiences. CHAPTER 8 Anxiety Disorders DAVID P....
-
As a manager of an airline company you want to learn the average weight of luggages checked in on a flight. From a sample of 1 6 luggages, you find the average to be 2 6 kg and the standard deviation...
-
The following information is available for Pyle Garage for March, Year 2: The following is a list of checks and deposits recorded on the books of Pyle Garage for March, Year 2: Other Information 1....
-
Using Gauss-Jordan elimination, invert this matrix ONLY 0 0 0 0 1
-
The chemisorptions of hydrogen on manganese is activated, but only weakly so. Careful measurements have shown that it precedes 35 per cent faster at 1000 K than at 600 K. What is the activation...
-
The adsorption of a gas is described by the Langmuir isotherm with K = 0.777 kPa-1 at 25C. Calculate the pressure at which the fractional surface coverage is (a) 0.20, (b) 0.75.
-
A certain solid sample adsorbs 0.63 mg of CO when the pressure of the gas is 36.0 kPa and the temperature is 300 K. The mass of gas adsorbed when the pressure is 4.0 kPa and the temperature is 300 K...
-
1. Sunday pichaiah, the CC.O of google belongs to ans level of managment
-
Key Graphics expects to finish the current year with the financial results indicated on the worksheet given below. Develop next years income statement and ending balance sheet using that information...
-
Discuss briefly the key elements of the revised COSO Framework

Study smarter with the SolutionInn App


