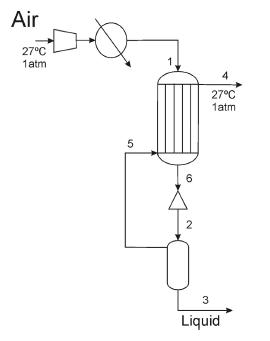
Atmospheric air is liquefied using a process as in Fig. P3.3. Air at 27C and 1 atm
Question:
Atmospheric air is liquefied using a process as in Fig. P3.3.
Air at 27C and 1 atm is compressed using 538.7 kJ/kg of initial air in a compressor whose efficiency is 80%. The compressed air is cooled down at constant pressure with the nonliquefied air. Next, it is expanded isenthalpically to obtain a liquid fraction of 0.05. Assume that all the heat losses take place in the heat exchanger. Compute the pressure of the air at the hot extreme of the heat exchanger (P1), the temperature of the nonliquefied air (T4), and the energy loss per kg of atmospheric air.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





