In the production of biodiesel from oil and methanol, the reactor conversion is given by this equation:
Question:
In the production of biodiesel from oil and methanol, the reactor conversion is given by this equation:
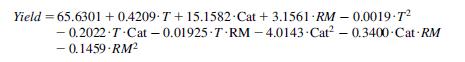
where the catalyst load is 1% of KaOH, Cat51, and the reaction takes place at 60C and 4 bar. The molar ratio between methanol and oil is 6.
The products from the reactor are distilled and 98% of the unconverted methanol is recovered. Determine the methanol fed to the system per 100 kmol/h of oil, and the purge fraction if the commercial oil contains 0.02 kmol of water per kmol of oil. Water leaves with the methanol. The catalyst is capable of handling 5 kmol of water per 100 kmol of the methanoloil mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





