The feedstock for the production of ammonia contains 0.5 mol of Ar per 100 mol of N2H2
Question:
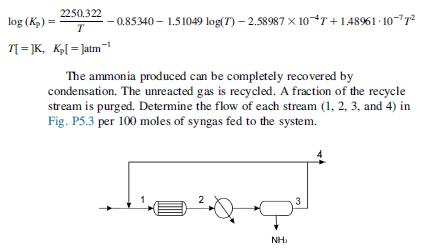
The feedstock for the production of ammonia contains 0.5 mol of Ar per 100 mol of N2H2 mixture. After mixing it with the recycle, the stream is fed to the converter. The syngas has stoichiometric proportions of nitrogen and hydrogen (N213H2). The gas also contains 5 moles of Ar per 100 moles of syngas. The reactor is operated at 200 atm and 740K isothermally where the equilibrium shown below takes place:
0:5N2 11:5H2’-NH3 The equilibrium constant (Kp) can be computed as a function of the temperature using the following equation:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





