Ignoring small electron binding energies and the very small mass of the neutrino, show that the mass
Question:
Ignoring small electron binding energies and the very small mass of the neutrino, show that the mass of a nucleus increases when it decays by electron capture if the Q-value of the decay is less than mec2 ? 0.511 MeV. Verify that this is the case for the electron capture decay of the longest-lived isotope of technetium:
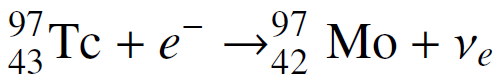
Transcribed Image Text:
97 Тс + e 43 Tc + e¯ →% 97 42 Мо + Ve
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
When a nucleus A Z decays to A1 Z 1 by electron captureenergy conservation gives where we have ig...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Electron-donating groups on benzene promote electrophilic aromatic substitution and lead preferentially to so-called ortho and para products over meta products, whereas electron-withdrawing groups...
-
The binding energies of K-shell and L-shell electrons in copper are 8.979 and 0.951 keV, respectively. If a Ka x ray from copper is incident on a sodium chloride crystal and gives a first-order Bragg...
-
The binding energy of a valence electron in a Li atom in the states 2S and 2P is equal to 5.39 and 3.54 eV respectively. Find the Rydberg corrections for S and P terms of the atom.
-
______________ is an approach to doing business that attempts to maximize an organization's competitiveness through the continual improvement of the quality of its products, services, people,...
-
You step onto a hot beach with your bare feet. A nerve impulse, generated in your foot, travels through your nervous system at an average speed of 110 m/s. How much time does it take for the impulse,...
-
A project has a life of 10 years and a payback period of 10 years. What must be true of project NPV?
-
Those who use their cell phones for a wide variety of activities are more likely to use the Internet to find health information, but perhaps this is only because of age (younger people are more...
-
On July 1, 2010, Brower Industries Inc. issued $32,000,000 of 10-year, 12% bonds at an effective interest rate of 13%, receiving cash of $30,237,139. Interest on the bonds is payable semiannually on...
-
3 00:26:52 Book Except for Multiple Choice O O business forms are types of separate, legal entities. limited partnerships sole proprietorships limited liabilities companies C corporations
-
A screen shot of this comma-separated file is shown below. Review this Source Data extract above and identify any issues with the raw data. (As needed, refer back to the ETL Overview document to help...
-
Verify the expressions for the Q-value in ? ? -decay and electron capture, eq. (17.34)? Q(B) = A(Z, A) A(Z + 1, A), Q(B+) = A(Z, A) A(Z 1, A) 2Ae Q(EC) = A(Z, A) - A(Z 1, A),
-
97% of naturally occurring calcium is calcium-40, 40 20 Ca. This may seem surprising, since if we use the semi-empirical mass formula to estimate the most stable nuclide with A = 40 we find Z 18....
-
Lucas County, Ohio presented the following table in its comprehensive annual financial report for the fiscal year ended December 31, 2010: Required a. Complete a horizontal common- size analysis. Use...
-
Given the following differential equation, dydx = sin ( x + y ) Find the following: ( a ) The substitution u = ( b ) The transformed differential equation dudx = ( c ) The implicit solution, given...
-
Consider the following type declarations TYPE Alinteger; A2 pointer to float; A3 pointer to integer; T1 structure (x: integer; } T2 structure (x: A1; next pointer to integer; } b float; } a :...
-
https://www.viddler.com/embed/82b62f65 Questions: How do companies decide where to locate their facilities? Why has just-in-time inventory control become a dominant production process used in the...
-
Adjusting Entries for Interest At December 31 of Year 1, Portland Corporation had two notes payable outstanding (notes 1 and 2). At December 31 of Year 2, Portland also had two notes payable...
-
We want to get an idea of the actual mass of 235U involved in powering a nuclear power plant. Assume that a single fission event releases 200 MeV of thermal energy. A 1,000 MWe electric power plant...
-
Case is a car assessor shop provides the car windows tinting. The shop ordered three different types of film from a manufacturer. According to the manufacturer, the dyed window tint film is the most...
-
How do the principles of (a) Physical controls and (b) Documentation controls apply to cash disbursements?
-
(a) Calculate the density of the atmosphere at the surface of Mars (where the pressure is 650 Pa and the temperature is typically 253 K, with a CO 2 atmosphere), Venus (with an average temperature of...
-
A large cylindrical tank contains 0.750 m 3 of nitrogen gas at 27 o C and 7.50 10 3 (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. What will be the...
-
Digesting fat produces 9.3 food calories per gram of fat, and typically 80% of this energy goes to heat when metabolized. (One food calorie is 1000 calories and therefore equals 4186 J.) The body...
-
Please note, kindly no handwriting. Q. Suppose a 3 year bond with a 6% coupon rate that was purchased for $760 and had a promised yield of 8%. Suppose that interest rates increased and the price of...
-
Be prepared to explain the texts comprehensive To illustrate the issues related to interest capitalization, assume that on November 1, 2016, Shalla Company contracted Pfeifer Construction Co. to...
-
On April 1, 2020. Indigo Company received a condemnation award of $473,000 cash as compensation for the forced sale of the company's land and building, which stood in the path of a new state highway....

Study smarter with the SolutionInn App


