(a) Calculate the density of the atmosphere at the surface of Mars (where the pressure is 650...
Question:
(b) Compare each of these densities with that of the earth€™s atmosphere, which is 1.20 kg/m3. Consult the periodic chart in Appendix D to determine molar masses.
In Appendix D:
Periodic Table of the Elements
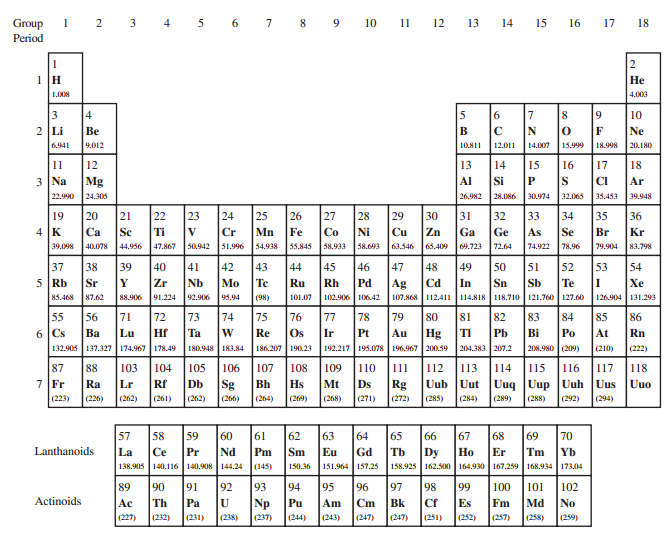
Transcribed Image Text:
4 5 6 7 8 9 10 11 12 13 14 Group 15 16 17 18 Period Не 4.003 1.008 4 2 Li 3 9. 10 Be Ne 9.012 12.011 14.007 15.999 20.180 6.941 10.811 18.998 EELE EE EEERBE 11 12 13 14 15 16 17 18 Mg 3 Na Si Al Ar 22.990 24.305 30.974 32.065 35.453 26.982 28.086 39.948 22 23 24 25 26 27 28 Fe Co 33 31 35 20 21 19 29 30 32 34 36 4 K Cr Ca Sc Mn Ni Cu Zn Ga Ge As Se Br Kr 79.904 44.956 47.867 51.996 54.938 58.933 58.693 65.409 69.723 72.64 83,798 39.098 40.078 50.942 55.845 63.546 74.922 78.96 39 47 Ag 102.906 106.42 46 50 37 38 40 41 42 43 44 45 48 49 51 52 53 54 5 Rb Nb 91.224 Mo Te Sr Zr Ru Rh Pd Cd In Sn Sb Te Xe 88.906 126.904 131.293 85.468 87.62 92.906 95.94 101.07 107.868 112.411 114.818 118.710 121.760 127.60 (98) 71 72 83 55 73 74 75 76 77 78 79 80 81 82 84 85 86 6 Cs 132.905 137.327174.967 178.49180.948 183.84 Os 192.217 195.078 196.967 200.59 204.383 207.2 Hg TI Ba Lu Hf Ta Re Ir Pt Au Pb Bi Po At Rn 208.980 (209) 186.207 190.23 (210) (222) EEE 87 7 Fr (223) 107 108 Sg (262) 88 103 104 105 106 109 110 11 112 113 14 115 116 117 118 Uub Uut Uuq Uup Uuh Ra Lr Rf Db Bh Mt Ds Rg Uus Uuo (226) (289) (294) (262) (261) (266) (264) (269) (268) (271) (272) (285) (284) (288) (292) 60 68 58 Ce 138.905 140.116140.908 144.24 (145) 61 62 65 66 Dy 158.925 162.500 164.930 167.259 168.934 173.04 69 57 59 63 64 67 70 Lanthanoids Tm La Pr Nd Pm Sm Gd Ь Er Yb 150.36 151.964157.25 90 Ac (232) 91 92 93 95 97 98 99 89 94 96 100 101 102 Actinoids Fm (257) Th Pa Np (237) Pu Am Cm Bk Cf Es Md No (247) (227) (231) (238) (244) (243) (247) (251) (252) (258) (259) EEEEEE
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
Identify We know the pressure and temperature and want to find the density of the gas The ideal gas ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

University Physics with Modern Physics
ISBN: 978-0321696861
13th edition
Authors: Hugh D. Young, Roger A. Freedman, A. Lewis Ford
Question Posted:
Students also viewed these Physics questions
-
Calculate the density of oxygen at STP using the ideal gas law.
-
Calculate the density of a white dwarf whose mass is equal to the Suns and whose radius is equal to the Earths. How many times larger than Earths density is this?
-
Calculate the density of states N(E) for a metal at energy E = 8.0eV and show that your result is consistent with the curve of figure.
-
Using the Internet, explore the most useful mobile applications for a business or industry of your choice. Also explore mobile applications for this business or industry that are not currently...
-
There has been a major global crisis, and your company's board of directors has announced that the company is going bankrupt. No one could have seen this one coming. Your CEO has called you in to his...
-
To what extent should managers appeal to social norms when planning the work of their staff? What are the benefits and what are the dangers?
-
What is the macrostructure of public organizations? LO.1
-
On August 1, 2011, Dr. Dana Hendley established Med, a medical practice organized as a professional corporation. The following conversation occurred the following February between Dr. Hendley and a...
-
Inc. Case 5.3 Facebook, The following excerpts are from the 2013 Form 10-K of Facebook, Inc.1 FACEBOOK, INC. CONSOLIDATED BALANCE SHEETS In millions, excapt for mumber of shares and par palue...
-
The following data report total, monthly U.S. book-store sales in millions of dollars fromJanuary 2016 to March 2019. (Go to https://www.census.gov/retail/index.html#mrts, find Monthly Retail Trade...
-
A cylindrical tank has a tight-fitting piston that allows the volume of the tank to be changed. The tank originally contains 0.110 m 3 of air at a pressure of 0.355 atm. The piston is slowly pulled...
-
A large cylindrical tank contains 0.750 m 3 of nitrogen gas at 27 o C and 7.50 10 3 (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. What will be the...
-
What are the different career opportunities in the cruise industry? LO.1
-
Discuss the Competitive Markets and Externalities simulations (both with and without policy interventions) . What impact do policy interventions have on the supply and demand equilibrium for a...
-
The best consultant to fix issue number one is Frederick Taylor who is credited with creating the scientific management movement (Lumen, n.d.). Since Taylor's work focused on how a process could be...
-
1. Which Pepsico products are growing faster than soft drinks (why) and by what percentage? 2. Why do the fastest growing products experience a more complex supply chain? Explain. 3. What are some of...
-
Use BLUF (Bottom Line UP Front) or Brief for answering the following questions: 1) There are a number of InfoSec frameworks / models available in industry. A. What is an InfoSec framework / model? B....
-
An introduction to organizational structure. Topics such as alternative organizational structures, the reciprocal relationship between multinational strategy and structure, and how recourses affect...
-
Let A be a 3 x 3 matrix with the property that the linear transformation x Ax maps R onto R. Explain why the transformation must be one-to-one.
-
On October 31 Juanita Ortega, owner of Outback Guide Service, received a bank statement dated October 30. Juanita found the following: 1. The checkbook has a balance of $2,551.34. 2. The bank...
-
A student is sitting on a frictionless rotating stool with her arms outstretched as she holds equal heavy weights in each hand. If she suddenly lets go of the weights, will her angular speed...
-
If two spinning objects have the same angular momentum, do they necessarily have the same rotational kinetic energy? If they have the same rotational kinetic energy, do they necessarily have the same...
-
If the earths climate continues to warm, ice near the poles will melt, and the water will be added to the oceans. What effect will this have on the length of the day? Justify your answer.
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


