Question: Starting from the MaxwellBoltzmann distribution, eq. (18.10) for the probability of finding a particle with energy E in a gas with temperature T, show that
Starting from the Maxwell–Boltzmann distribution, eq. (18.10)
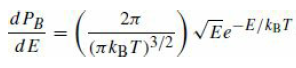
for the probability of finding a particle with energy E in a gas with temperature T, show that the most probable speed for a particle is v̅ = √2kBT/m, but that the average speed of a particle is 〈v〉 = √8kBT/πm. Verify that the most probable speed for a neutron at T = 20◦C is ∼2200m/s.
dPB VEe-E/kBT (7 kB T)3/2 dE
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
First we change variable from E to v using E mv 2 2 and dE mv dv To ... View full answer

Get step-by-step solutions from verified subject matter experts


