In 1953, Stanley Miller and Harold Urey performed an experiment at the University of Chicago to test
Question:
In 1953, Stanley Miller and Harold Urey performed an experiment at the University of Chicago to test the hypothesis that the conditions of the early Earth would have favored the formation of larger, more complex organic molecules from basic precursors. The experiment, shown in Figure 1, consisted of sealing basic organic chemicals (representing the atmosphere of the primitive Earth) in a flask, which was exposed to electric sparks (to simulate lightning) and water vapor.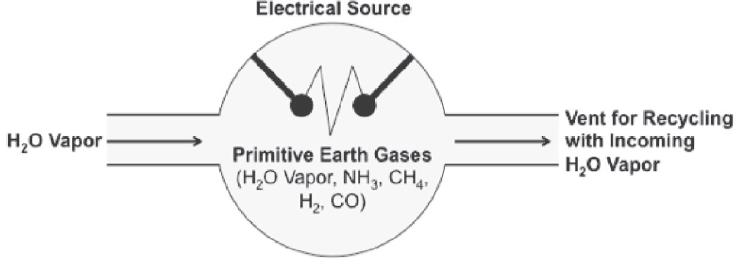
After one day of exposure, the mixture in the flask had turned pink in color, and later analysis showed that at least 10% of the carbon had been transformed into simple and complex organic compounds including at least 11 different amino acids and some basic sugars. No nucleic acids were detected in the mixture.
Some amino acids, such as cysteine and methionine, could not be formed in this experiment. Which of the following best explains why these molecules could not be detected?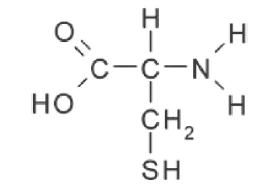
(A) The chemical reactions necessary to create amino acids such as cysteine and methionine require more energy than the simulated lightning provided in the experiment.
(B) The chemical reactions necessary to create amino acids such as cysteine and methionine require enzymes for catalysis to occur, which were not included in the experiment.
(C) Sulfur-based compounds were not included in the experiment.
(D) Nitrogen-based compounds were not included in the experiment
Step by Step Answer:

The Princeton Review AP Biology Premium Prep 2023
ISBN: 9780593450659
2023 Edition
Authors: The Princeton Review





