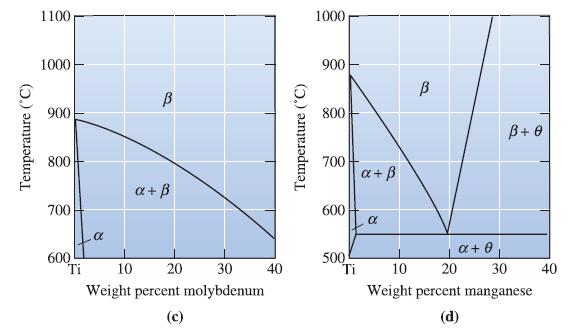
A Ti-Mn alloy is composed of 20 wt% manganese and is kept at 550C. What phase(s) are
Question:
A Ti-Mn alloy is composed of 20 wt% manganese and is kept at 550°C. What phase(s) are present? See Figure 14-8(d).
Transcribed Image Text:
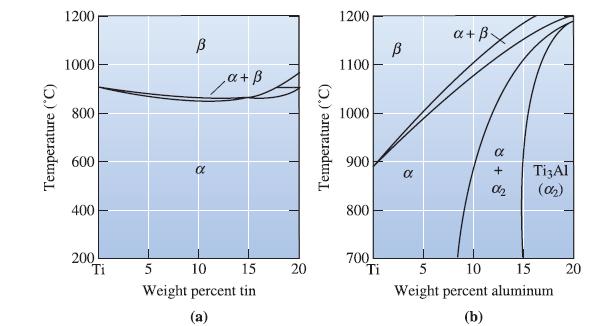
Temperature (°C) 1200 1000 800 600 400 200 Ti В α ‚a + ß 5 10 15 Weight percent tin (a) 20 Temperature (°C) 1200 1100 1000 900 800 700 Ti В 8 a+ß- α + Ti3Al 02 (02₂) 5 10 15 Weight percent aluminum (b) 20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
The phases present include i a ferri...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Set up iterated integrals for both orders of integration. Then evaluate the double integral using the easier order and explain why its easier. D is bounded by y = x 3 , y = 2x + 4, x = 0 6x2 dA, D
-
Some hypothetical alloy is composed of 12.5 wt% of metal A and 87.5 wt% of metal B. If the densities of metals A and B are 4.27 and 6.35 g/cm 3 , respectively, whereas their respective atomic weights...
-
Some hypothetical alloy is composed of 12.5 wt% of metal A and 87.5 wt% of metal B. If the densities of metals A and B are 4.27 and 6.35 g/cm3, respectively, whereas their respective atomic weights...
-
Use the Ratio Test to determine the values of x 0 for which each series converges. 00 X 2k 2 k=1 k
-
1. Also the percentage increase for each occupational category. 2. The Excel file Store and Regional Sales Database provides sales data for computers and peripherals showing the store identification...
-
Does the problem facing the owners of Pet Groomer on Wheels seem suited for going through the steps for group problem solving? Or, what other problem-solving technique would you recommend? Take the...
-
how to test a slope for statistical significance
-
1. Should you discuss the matter first with Troy before responding to Joyce? Explain. 2. Assume Kristen is a Certified Management Accountant and member of the Institute of Management Accountants. As...
-
A project has annual cash flows of $4,500 for the next 10 years and then $8,000 each year for the following 10 years. The IRR of this 20-year project is 10.91%. If the firm's WACC is 10%, what is the...
-
Young, innovative, or high-tech firms often adopt the strategy of ignoring history or attempting to do something radically new. In what ways might this strategy help them? In what ways might this...
-
A 5182-O aluminum alloy part that had been exposed to salt water showed severe corrosion along the grain boundaries. Explain this observation based on the expected phases at room temperature in this...
-
Would you expect a 2024-T9 aluminum alloy to be stronger or weaker than a 2024-T6 alloy? Explain.
-
An asset is bought by Placer Ltd for a project at a cost of $\$ 25,000$. The asset will be used for four years before being disposed of for $\$ 5,000$. Tax-allowable depreciation is available at $25...
-
Aircraft \(B\) has a constant speed of \(150 \mathrm{~m} / \mathrm{s}\) as it passes the bottom of a circular loop of 400-m radius. Aircraft \(A\) flying horizontally in the plane of the loop passes...
-
A small inspection car with a mass of \(200 \mathrm{~kg}\) runs along the fixed overhead cable and is controlled by the attached cable at \(A\). Determine the acceleration of the car when the control...
-
An aircraft \(P\) takes off at \(A\) with a velocity \(v_{0}\) of \(250 \mathrm{~km} / \mathrm{h}\) and climbs in the vertical \(y^{\prime}-z^{\prime}\) plane at the constant \(15^{\circ}\) angle...
-
If each resistor in Figure P31.75 has resistance \(R=5.0 \Omega\), what is the equivalent resistance of the combination? Data from Figure P31.75 wwwwww wwwww www www wwwww
-
Identify the proper point to recognize expense for each of the following transactions. a. Kat Inc. purchases on credit six custom sofas for \(\$ 800\) each in June. Two of the sofas are sold for \(\$...
-
Discuss five methods commonly used to increase cash inflows.
-
Three successive resonance frequencies in an organ pipe are 1310, 1834, and 2358 Hz. (a) Is the pipe closed at one end or open at both ends? (b) What is the fundamental frequency? (c) What is the...
-
Use the matrix inverse and the matrix division method to solve the following set for x and y in terms of c: 4cx + 5y = 43 3x 4y = -22
-
The currents i 1 , i 2 , and i 3 in the circuit shown in Figure P12 are described by the following equation set if all the resistances are equal to R. Here 1 and 2 are applied voltages; the other...
-
The equations for the armature-controlled dc motor shown in Figure P13 follow. The motors current is i, and its rotational velocity is 2. where L, R, and I are the motors inductance, resistance, and...
-
ACC 2 0 2 Milestone One: Operational Costs Data Appendix You plan to open a small business for manufacturing pet collars, leashes, and harnesses. You have found a workshop space you can use for...
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...

Study smarter with the SolutionInn App


