Balance the following oxidationreduction reactions that occur in acidic solution using the half-reaction method. a. Cu(s) +
Question:
Balance the following oxidation–reduction reactions that occur in acidic solution using the half-reaction method.
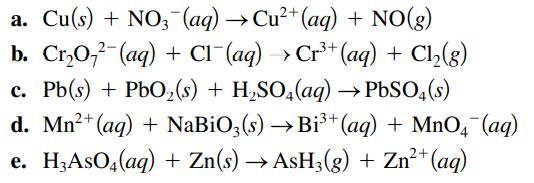
Transcribed Image Text:
a. Cu(s) + NO3(aq) → Cu²+ (aq) + NO(g) b. Cr₂O72 (aq) + Cl¯(aq) → Cr³+ (aq) + Cl₂(g) c. Pb(s) + PbO₂ (s) + H₂SO4(aq) → PbSO4(s) d. Mn²+ (aq) + NaBiO3(s) →Bi³+ (aq) + MnO4 (aq) e. H,AsO4(aq) + Zn(s) → AsH3(g) + Zn²+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
a Cus NO3aq Cuaq NOg Halfreaction Oxidation Cus Cuaq Reduction NO3aq N...View the full answer

Answered By

Hande Dereli
Enthusiastic tutor, skilled in ACT and SAT tutoring. Raised one student's score on the SATs from 1100 combined to 1400. Graduated with a 3.9 GPA from Davidson College and led a popular peer tutoring group for three years. Scored in the top 0.06% in the nation on the SATs. The real reason I'm the one to help you nail the test? Results. Clients invariably praise my ability to listen and communicate in a low-stress, fun way. I think it's that great interaction that lets me raise retest SAT scores an average of 300 points.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Balance each of the following oxidation reduction reactions by using the oxidation states method. a. C2H6(g) + O2(g) CO2(g) + H2O(g) b. Mg(s) + HCl(aq) Mg2+(aq) + Cl2(aq) + H2(g) c. Cu(s) + Ag+(aq)...
-
Balance these redox reactions that occur in aqueous solution. Use whatever water-derived species is necessary; there may be more than one correct balanced equation. a. CrO3 + Ni2+ ( Cr3+ + Ni3+ b....
-
Balance these redox reactions that occur in aqueous solution. Use whatever water-derived species is necessary; there may be more than one correct balanced equation. a. ClO + Ti2+ ( Ti4+ + Cl b. BrO3...
-
My division had another great year last year. We all worked hard, and the results were there. But again we got no reward for our hard work. It's very frustrating. - Division Manager, General Products...
-
What problem may exist in determining the amount realized for an investor who exchanges common stock of a publicly traded corporation for a used building? How is the problem likely to be resolved?
-
Zip Shuttle Service operates airport shuttle vans in 12 large cities: Los Angeles, San Diego, San Francisco, Phoenix, Las Vegas, Houston, Dallas, Chicago, New York City, Washington DC, Miami, and...
-
The standard mix to produce one unit of product is as follows: Material A = 60 units @ Rs. 15 per unit = Rs. 900 Material B = 80 units @ Rs. 20 per unit = Rs. 1,600 Material C = 100 units @ Rs. 25...
-
The information in the table is from the statement of cash flows for a company at four different points in time (A, B, C, and D). Negative values are presented in parentheses. InstructionsFor each...
-
Sheffield Company's manufacturing overhead budget shows total variable costs of $ 2 4 5 , 5 2 0 and total fixed costs of $ 2 0 0 , 8 8 0 . Total production in units is expected to be 1 8 6 , 0 0 0 ....
-
On January 1. Ruiz Company issued bonds as follows: Face Value: Number of Years: Stated Interest Rate: Interest payments per year 500,000 15 7% Required: 1) Calculate the bond selling price given the...
-
Specify which of the following equations represent oxidation reduction reactions, and indicate the oxidizing agent, the reducing agent, the species being oxidized, and the species being reduced. a....
-
A 30.0-mL sample of an unknown strong base is neutralized after the addition of 12.0 mL of a 0.150 M HNO 3 solution. If the unknown base concentration is 0.0300 M, give some possible identities for...
-
The bent rod ABDE is supported by ball-and-socket joints at A and E and by the cable DF. If a 60-lb load is applied at C as shown, determine the tension in the cable. in. 7 in 60 lb ll in 10 in x 14...
-
Gilbert Canned Produce (GCP) packs and sells three varieties of canned produce: green beans; sweet peas; and tomatoes. The company is currently operating at 82 percent of capacity. Worried about the...
-
Apply at least two of the theories (of your choice) to your personal experience? The theories are Leader-Member Exchange Theory (LMX Model), the Situational Leadership Model, the Contingency Model...
-
Game theory is used in economics, social science and computer science to understand and predict the behaviour of people and intelligent entities. In project management and business scenarios, it can...
-
During a chemistry lab, you take a 0.2 kg sample of ice and put it in a beaker with a thermometer. You then place the beaker with the ice on a hot plate, and turn on the hot plate. This hot plate...
-
Selected information from Carla Vista Ltd.'s statement of financial position and statement of income is as follows: Carla Vista Ltd. Statement of Financial Position (partial) December 31 2024 2023...
-
1. If we were to look at weekly rather than hourly pay, and included the effects of overtime, what do you think would happen to the pay differentials in Table (a)? 2. In Table (b), which of the...
-
The relationship described in question 7 does not always appear to hold. What factors, besides the number of firms in the market, might affect margins?
-
A copper penny can be dissolved in nitric acid but not in hydro chloric acid. Using reduction potentials from the book, show why this is so. What are the products of the reaction? Newer pennies...
-
You want to plate out nickel metal from a nickel nitrate solution onto a piece of metal inserted into the solution. Should you use copper or zinc? Explain.
-
Galvanic cells harness spontaneous oxidationreduction reactions to produce work by producing a current. They do so by controlling the flow of electrons from the species oxidized to the species...
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


