Calculate the equilibrium concentrations of NH 3 , Cu 2+ , Cu(NH 3 ) 2+ , Cu(NH
Question:
Calculate the equilibrium concentrations of NH3, Cu2+, Cu(NH3)2+, Cu(NH3)22+, Cu(NH3)32+, and Cu(NH3)42+ in a solution prepared by mixing 500.0 mL of 3.00 M NH3 with 500.0 mL of 2.00 × 10-3 M Cu(NO3)2. The stepwise equilibria are
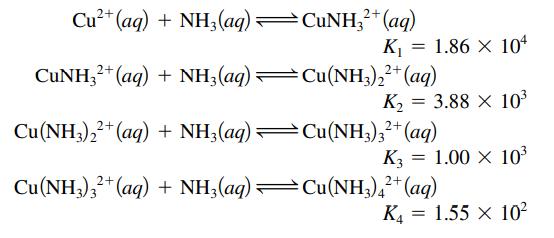
Transcribed Image Text:
Cu²+ (aq) + NH3(aq) CUNH3²+ (aq) + NH3(aq) —Cu(NH3)₂²+ (aq) CuNH32+ (aq) 2+ K₁ = 1.86 x 104 2+ K₂ Cu(NH3)22+ (aq) + NH3(aq) —Cu(NH3)3²+ (aq) = 3.88 × 10³ K3= 1.00 x 10³ Cu(NH3)3²+ (aq) + NH3(aq) —Cu(NH3)4²+ (aq) K4 = 1.55 X 10²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
Solution The equilibrium concentrations of NH3 0200 M Cu2 0025 M CuNH32 0150 M ...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Calculate [HY3-] in a solution prepared by mixing 10.00 mL of 0.010 0 M VOSO4, 9.90 mL of 0.010 0 M EDTA, and 10.0 mL of buffer with a pH of 4.00.
-
Calculate the pH of a solution prepared by mixing 0.0800 mol of chloroacetic acid plus 0.0400 mol of sodium chloroacetate in 1.00 L of water. (a) First do the calculation by assuming that the...
-
A solution prepared by mixing 10 mL of a 0.10 M solution of the R enantiomer and 30 mL of a 0.10 M solution of the S enantiomer was found to have an observed specific rotation of +480. What is the...
-
Spherical refracting surfaces an object O stands on the central axis of a spherical refracting surface. For this situation, each problem in Table 34-5 refers to the index of refraction n1 where the...
-
Valesquez Ranches, Inc., wishes to use a new truck fueled by compressed natural gas that costs $80,000. The ranch intends to operate the truck for five years, at the end of which time it is expected...
-
In distance-vector routing, bad news (increase in a link metric) will propagate slowly. In other words, if a link distance increases, sometimes it takes a long time for all nodes to know the bad...
-
Define expert systems, If/Then rules, and expert systems shell. Explain how expert system rules are created. Summarize the three major disadvantages of expert systems, and assess the future of these...
-
A fitness magazine claims that the mean cost of a yoga session is no more than $14. You work for a consumer advocacy group and are asked to test this claim. You find that a random sample of 32 yoga...
-
Problem 15-7 (Algo) Prepare a Statement of Cash Flows (LO15-1, LO15-2] [The following information applies to the questions displayed below.) Comparative financial statements for Weaver Company...
-
The following trial balance has been extracted from the books of Walrus plc as at 31 March 2020: The following information is also available: 1. Non-depreciable land was valued at 300,000 on 31 March...
-
The K sp of Al(OH) 3 is 2 10 -32 . At what pH will a 0.2-M Al 3+ solution begin to show precipitation of Al(OH) 3 ?
-
The solubility of copper(II) hydroxide in water can be increased by adding either the base NH 3 or the acid HNO 3 . Explain. Would added NH 3 or HNO 3 have the same effect on the solubility of silver...
-
For the same situation as in Exercise 4.5, which of the following is always true of the name resolution process, assuming that all name servers are configured correctly and no messages are lost? A....
-
What is a transistor, and what are its types?
-
Discuss the emerging role of nanotechnology in electrical engineering, focusing on its applications in enhancing electrical components like batteries, supercapacitors, and sensors.Explore the...
-
1. As resistors are added in parallel to an existing circuit, what happens to the voltage drop measured across each resistor? 2. In the circuit shown on the right, which path (left or right) will...
-
Establish identity. cot(20) tan 0) 5(cot 0
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
Draw a tetrahedral representation of (S)-2-pentanol (2-hydroxypentane).
-
Assign R or S configuration to the chirality center in the following molecular model of the amino acid methionine (blue = N, yellow =S):
-
One of the following molecules (a)(d) is D-erythrose 4-phosphate, an intermediate in the Calvin photosynthetic cycle by which plants incorporate CO 2 into carbohydrates. If D-erythrose 4-phosphate...
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...
-
Give a breakdown of all the intangible assets with the values shown in the statement of financial position of Unilever in 2022.
-
1-The yield to maturity will be greater than the coupon rate when a bond is selling at a premium. Select one: a. False b. True 2-Which one of the following would have the greatest present value,...

Study smarter with the SolutionInn App


