Assign R or S configuration to the chirality center in the following molecular model of the amino
Question:
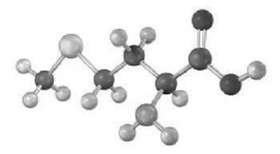
Assign R or S configuration to the chirality center in the following molecular model of the amino acid methionine (blue = N, yellow =S):
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (18 reviews)
Fortunately methioni...View the full answer

Answered By

Jinah Patricia Padilla
Had an experience as an external auditor in Ernst & Young Philippines and currently a Corporate Accountant in a consultancy company providing manpower to a 5-star hotel in Makati, Philippines, Makati Diamond Residences
5.00+
120+ Reviews
150+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Assign R or S configuration to the chirality center in each of the followingmolecules: CH (c) . (b) (a) 2 "- HS efpo -- CH-
-
A Assign R or S configuration to the chirality centers in the following molecules (blue =N): (b) (a) Adrenaline Serine
-
Assign R or S configuration to the following molecule, write the product you would expect from SN2 reaction with NaCN, and assign R or S configuration to the product (yellow-green =Cl):
-
Bob was waiting at the crosswalk for the light to turn green. As he stood there, a car that was stopped in the road next to him suddenly exploded, and Bob was injured by the blast. A defect in the...
-
Consider a 6.0-g steel nail 8.0 cm long and a hammer that exerts an average force of 600 N on the nail when it is being driven into a piece of wood. The nail becomes warmer. Show that the increase in...
-
The following draft financial statements are available for Sipfalor plc for the year ended 31 May 2018: Statement of financial position at 31 May 2018. Statement of profit or loss for the year to 31...
-
how to standardize slopes and interpret them
-
Ambrose Corporation owns 75 percent of Kroop Company's common stock, acquired at underlying book value on January 1, 20X4. At the acquisition date, the book values and fair values of Kroop's assets...
-
Suppose that the index model for stocks A and B is estimated from excess returns with the following results: R A = 2 . 2 0 % + 0 . 8 0 R M + e A R B = - 2 . 2 0 % + 1 . 2 0 R M + e B M = 2 4 8 ; R -...
-
According to data from the U.S. Department of Energy, the average retail price of regular gasoline rose from $1.16 in 1990 to $2.52 in 2015, a 117% increase. a. Other things equal, describe the...
-
Draw a tetrahedral representation of (S)-2-pentanol (2-hydroxypentane).
-
One of the following molecules (a)(d) is D-erythrose 4-phosphate, an intermediate in the Calvin photosynthetic cycle by which plants incorporate CO 2 into carbohydrates. If D-erythrose 4-phosphate...
-
Design second-order active filters that meet the following requirements. Simulate your designs in Multisim to validate the requirements Low pass 50 k Wo 0 (rad/s) 3 1 Constraints de gain of 60 dB
-
How do emergent properties within teams, such as synergy and collective intelligence, manifest and influence team performance, and what factors contribute to their development and sustenance?
-
Think about your workplace, organization, or industry; if you are not currently working, think about previous employment or a job that you are aiming for. The broader your perspective, the more...
-
Choose any global organization that successfully undertook a strategic transformation to adapt to changing market dynamics and sustain its competitive advantage. Examine the company's challenges,...
-
In examining C&C Sports through the lens of a SWOT analysis, several key factors come to light. The strengths of the company are evident in its established brand reputation and a loyal customer base...
-
A perfectly insulated container initially contains 0.2 kg of ice at -15 C. Now we add water at 30 C, but only the minimum amount needed to barely melt all the ice. Find the net entropy change of the...
-
Why is champagne served in fluted glasses? LO.1
-
Kims Konstructions has assembled the following data for a proposed straw-reinforced brick maker (SRBM): SRBM Cost: $26,000 Life: 5 years Revenue (p.a.) $11,000 Operating Expenses (p.a.) $3,000...
-
The graph shows PV/RT for carbon dioxide at three different temperatures. Rank the curves in order of increasing temperature. (a) C (b) A (c) B (d) C PV/RT 2. 1.6- 1.2- 0.8- 0.4- 0- 0 200 400 600...
-
How would you differentiate between the compounds in each of the following pairs? (a) p-ethylbenzoic acid and ethyl benzoate by IR spectroscopy (b) N-methylpropanamide and N-ethyl acetamide by proton...
-
Which of the two isomers in each of the following sets should have the greater basicity at the carbonyl oxygen? Explain. H,C CHCHC- OCH or HCC-o-CH CH CH
-
Explain why ethyl acetoacelate (ethyl 3-oxobutanoate, pKa = 10.7) are much more acidic than ordinary esters. (To answer this question. you must first identify the acidic hydrogen in each of these...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and Fabrication. It started, completed, and...
-
Horizontal Analysis The comparative accounts payable and long-term debt balances of a company are provided below. Current Year Previous Year Accounts payable $47,286 $63,900 Long-term debt 85,492...
-
On January 1, Year 1, Price Company issued $140,000 of five-year, 7 percent bonds at 97. Interest is payable annually on December 31. The discount is amortized using the straight-line method. Record...

Study smarter with the SolutionInn App


