Carbon tetrachloride (CCl 4 ) and benzene (C 6 H 6 ) form ideal solutions. Consider an
Question:
Carbon tetrachloride (CCl4) and benzene (C6H6) form ideal solutions. Consider an equimolar solution of CCl4 and C6H6 at 25οC. The vapor above the solution is collected and condensed. Using the following data, determine the composition in mole fraction of the condensed vapor.
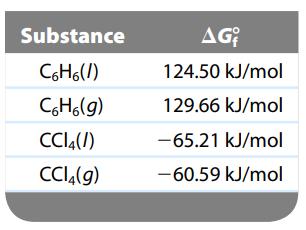
Transcribed Image Text:
Substance C6H6(/) C6H6(g) CC14(/) CC14(g) AG 124.50 kJ/mol 129.66 kJ/mol -65.21 kJ/mol -60.59 kJ/mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Solution The composition in mole fraction of the conden...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
An equimolar solution of benzene and toluene is totally evaporated at a constant temperature of 90C. What are the pressures at the beginning and end of the vaporization process? Assume an ideal...
-
The diffusion coefficient of glucose in water at 25 C is 6.73 10 10 m 2 s 1 . Estimate the time required for a glucose molecule to undergo a root-mean-square displacement of 5.0mm.
-
Using the following data determine the equation of the regression model. How many independent variables are there? Comment on the meaning of these regression coefficients. Predictor .. Coefficient...
-
How could sales force automation affect salesperson productivity, marketing management, and competitive advantage?
-
How does each of the following events affect the risk of a liquidity trap? a. The central bank decides to push long-run inflation to zero. b. The neutral real interest rate rises. c. The government...
-
In problem, graph each function using the techniques of shifting, compressing or stretching, and reflections. Identify any intercepts on the graph. State the domain and, based on the graph, find the...
-
Construct the cost matrix. Provide rationales for each cost.
-
What is the role of a chief security officer, and why is this organizational role a relatively new one?
-
The following data items are maintained in a company's database for each fixed asset item that it owns: fixed asset number, fixed asset description, fixed asset classification, location, responsible...
-
As the in-charge senior auditor on the audit engagement for JA Tire Manufacturing for the year ended December 31, 2019, you are responsible for performing risk assessment procedures related to the...
-
Tissue engineering involves the development of biological substitutes that restore or improve tissue function, Once manufactured, engineered organs can be implanted and grow within the patient,...
-
A 0.400-M solution of ammonia was titrated with hydrochloric acid to the equivalence point, where the total volume was 1.50 times the original volume. At what pH does the equivalence point occur?
-
Conduit Corporation has 45 current employees: 5 managers and 40 non-managers. The average wage paid is $250 per day for non-managers. The company has just finished negotiating a new employee contract...
-
What's your favourite way to promote and build interpersonalwellness? How do you nurture your relationships? How do you build friendships? How do you show your loved ones that you care? Share your...
-
What is the best way to summary this formation? IMC Communication Objectives Increase from 0% to 75% of Millennials in the Dallas-Fort Worth area, ages 22 - 34, with a four-year college degree,...
-
Branding is very important to marketing, that is while most businesses focus on brands that attract customer's interest. Store brand is unique to a particular store compared to national brand . A...
-
Do you think that your score accurately reflects your global mindset? Why or why not? What, if anything, is missing from the assessment? How do you think that having a higher global mindset will help...
-
Healthcare is an ever-changing industry that requires healthcare organizations to align with those changes or risk being left behind. With the advances being made in technology, every corner seems to...
-
Solve the equation for the indicated variable. Use logarithms with the appropriate bases. log A = log B - C log x, for A
-
The following selected accounts and normal balances existed at year-end. Notice that expenses exceed revenue in this period. Make the four journal entries required to close the books: Accounts...
-
For the half-cell reaction AgBr(s) + e Ag(s) + Br (aq), E o = +0.0713 V. Using this result and G f (AgBr, s) = 96.9 kJ mol 1 , determine G o f (Br, aq).
-
For the half-cell reaction Hg 2 Cl 2 (s) + 2e 2Hg(l) + 2Cl (aq), E o = +0.26808 V. Using this result and G o f (Hg 2 Cl 2 , s) 210.7 kJ mol -1 , = determine G o f (Cl , aq).
-
Determine the half-cell reactions and the overall cell reaction, calculate the cell potential, and determine the equilibrium constant at 298.15K for the cell Is the cell reaction spontaneous as...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...

Study smarter with the SolutionInn App


