Consider a galvanic cell based on the following theoretical half-reactions: What is the value of G
Question:
Consider a galvanic cell based on the following theoretical half-reactions:
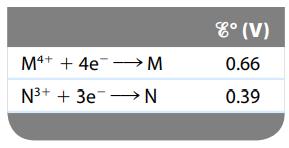
What is the value of ΔGο and K for this cell?
Transcribed Image Text:
M4+ + 4e→→→→→M N³+ + 3e N 8° (V) 0.66 0.39
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Its under conditions as well as the equilibrium constant K We know that this is a galvanic cell and ...View the full answer

Answered By

Dudhat Vaidehi
I tutored mostly elementary school students privately after school and during the summer. We met in their homes or at the public library. I charged an hourly fee, and I provided any necessary materials.
Having taught in special education in two local schools for many years meant that I had contact with a lot of parents of special needs students. I never had to advertise — word of mouth was how most folks knew of me. At one point I did have a website, but didn't utilize it much. I stayed very busy, especially in the summers, and always had a full schedule. I typically met with each student's teacher in order to get an idea of what the focus of my instruction/remediation should be. Becoming familiar with the student's learning style(s) was also very helpful. Often parents would share records and test results with me. After each tutoring session, I documented the student’s progress and gave parents written updates, as well as phone calls or emails as needed.
While my students and I certainly utilized technology and the internet often during our sessions, I never tutored online or for any tutoring company, so am not familiar with the curriculums or methods used in those settings.
Tutoring one on one was very enjoyable and rewarding. My students and I had fun, and grew quite fond of one another. The extra income was a bonus. I had to retire from tutoring due to a physically handicapping disease, and miss my students very much.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the standard galvanic cell based on the following half reactions Cu2+ + 2e- Cu Ag+ + e- Ag The electrodes in this cell are Ag(s) and Cu(s). Does the cell potential increase, decrease, or...
-
Consider the galvanic cell based on the following halfreactions: b. Calculate ÎGo and K for the cell reaction at 25oC. c. Calculate cell at 25oC when [Zn2+] = 0.10 M and [Fe2+] = 1.0 Ã...
-
Consider the galvanic cell based on the following halfreactions: b. Calculate ÎGo and K for the cell reaction at 25oC. c. Calculate cell at 25oC when [Au3+] = 1.0 Ã 102 M and [Tl+] = 1.0...
-
Route Canal Shipping Company has the following schedule for aging of accounts receivable: AGE OF RECEIVABLES APRIL 30, 2001 a. Fill in column (4) for each month. b. If the firm had $1,440,000 in...
-
Link from the text Web site to the online Economic Report of the President and get annual data on real GDP and real investment. Calculate the growth rates of the two variables for each year since...
-
In problem, find the average rate of change of f: (a) From 1 to 2 (b) From 0 to 1 (c) From 2 to 4 f(x) = 8x 2 x
-
Using the training set, and the cost matrix, develop a CART model for predicting Churn. Call this Model 2.
-
The following information (presented in thousands) is available for the Cumberland County Utility Enterprise Fund during the current year: 1. The beginning balance for cash and cash equivalents was...
-
Tristar Matutacturing produces two types of battery-operated toy soldiers infantry and special forces. The soldiers are produced by using one continuous process. Four activities have been identified...
-
Adam received a bonus of $6,000 from his employer. Which one of the following federal income withholding tax amounts is not in accordance with IRS rules regarding supplemental wage payments? Adam...
-
When the environment is contaminated by a toxic or potentially toxic substance (for example, from a chemical spill or the use of insecticides), the substance tends to disperse. How is this consistent...
-
In the electrolysis of a sodium chloride solution, what volume of H 2 (g) is produced in the same time it takes to produce 257 L Cl 2 (g), with both volumes measured at 50. C and 2.50 atm?
-
Complete the following table by indicating whether the listed transactions would improve, worsen, or have no effect on the financial ratios listed below. Consider each transaction independently. The...
-
How have you maintained your medical billing skills over the past 12 months? Include any courses or learning opportunity you used to build your current knowledge base. How did these skills help you?...
-
1. What issues does Bob Holland face as he takes over as CEO of Ben & Jerry's? Which are the most important? 2. Where is the market headed? What are the competitive influences and compare the...
-
Do you think there is a difference between diversity management and affirmative action? Provide an explanation for your response. Support your response with APA cited references. Response: Diversity...
-
1. In what ways do practical and statistical significance work together to help us understand program effects? Can one be important to aprogram evaluator withoutthe other? If so, how? If not, why...
-
How do IT metrics, measurements, productivity, and efficiency work together? Make sure you explain each word.Make sure to pick out two or three specific IT data and measures. Also, back up what you...
-
Solve the equation. Give solutions in exact form. 3 log, V2x2 2
-
The production budget of Artest Company calls for 80,000 units to be produced. If it takes 30 minutes to make one unit and the direct labor rate is $16 per hour, what is the total budgeted direct...
-
Compound F, a hydrocarbon with M + = 96 in its mass spectrum, undergoes reaction with HBr to yield compound G. Propose structures for F and G, whose 13 C NMR spectral data follow. Compound 1:...
-
3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 ?. Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be...
-
A 13C NMR spectrum of commercially available 2, 4-pentanediol, shows five peaks at 23.3, 23.9, 46.5, 64.8, and 68.1 ?. Explain. CHCH-CHCH3 2,4-Pentanediol
-
Mass LLp developed software that helps farmers to plow their fiels in a mannyue sthat precvents erosion and maimizes the effoctiveness of irrigation. Suny dale paid a licesnsing fee of $23000 for a...
-
Average Rate of Return The following data are accumulated by Lone Peak Inc. in evaluating two competing capital investment proposals: 3D Printer Truck Amount of investment $40,000 $50,000 Useful life...
-
4. (10 points) Valuation using Income Approach An appraiser appraises a food court and lounge and provides the following assessment: o O The building consists of 2 floors with the following (6)...

Study smarter with the SolutionInn App


