A 13C NMR spectrum of commercially available 2, 4-pentanediol, shows five peaks at 23.3, 23.9, 46.5, 64.8,
Question:
A 13C NMR spectrum of commercially available 2, 4-pentanediol, shows five peaks at 23.3, 23.9, 46.5, 64.8, and 68.1 ?. Explain.
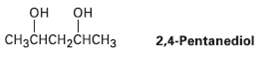
Transcribed Image Text:
он он CHзCнсH-CHCH3 2,4-Pentanediol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
Commercial 24pentanediol is a mixture of three stereoisomers RR SS and RS The meso isomer sh...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectraldata. 100 80 60 40 20 aly 120 10 40 60 80 100 140 m/z TMS 200 180...
-
A compound C6H14O has the 13C NMR spectrum shown in Figure 15.7. Its mass spectrum has a prominent peak at m/z 31. Suggest a reasonable structure for this compound.
-
A compound (C3H7ClO2) exhibited three peaks in its 13C NMR spectrum at 46.8 (CH2), 63.5 (CH2), and 72.0 ppm (CH). Excluding compounds that have Cl and OH on the same carbon, which are unstable, what...
-
Using the SEDAR database, find the most recent annual reports for two Canadian retailers (e.g., Loblaw, Rona, Danier Leather). Required: a. Based on the information provided in the companies audited...
-
In rapidly developing economies --- such as India and South Korea --- conglomerates are far more common than they are in the US and Western Europe. Use the BCG growth/share matrix to explain why this...
-
What are the emf and internal resistance of the battery in FIGURE P28.46? Open: 1.636 A A Closed: 1.565 A 50 100 FIGURE P28.46
-
How can the grapevine be used to a companys advantage?
-
There are some 200 economic integration agreements in effect around the world already, far more than even a few years ago. Virtually every country is now party to one or more free trade agreements....
-
PNG electric company manufactures a number of electric products. Rechargeable light is one of the PNGs products that sells for $180/unit. Total fixed expenses related to rechargeable electric light...
-
You are the Owners project manager on an electrical upgrade project. Existing overhead power lines are being buried in a duct bank to hide them from view in an area of the town that is being...
-
3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 ?. Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be...
-
Carboxylic acids (RCO 2 H) react with alcohols (R?OH) in the presence of an acid catalyst. The reaction product of propanoic acid with methanol has the following spectroscopic properties. Propose a...
-
Zen began a new consulting firm on January 5. Following is a financial summary, including balances, for each of the company's first five transactions (using the accounting equation form). Identify...
-
Claxton, Inc. paid a dividend of $ 0 . 9 5 per common share every December from 2 0 0 9 through 2 0 2 3 . The dividend is expected to continue at that level in 2 0 2 4 and 2 0 2 5 . In 2 0 2 6 and...
-
1. Who are two (2) specific examples of effective leaders (who you know personally) who have impacted your life? 2. What made them "effective" leaders? What many specific traits did these leaders...
-
pr Hwk12 Consider the differebtiable function F(x) whose instantaneous rates are given by the table of values below: I 3.00 3.50 4.00 4.50 5.00 5.50 6.00 6.50 7.00 F'(x) 21.00 27.50 35.00 43.50 53.00...
-
A stone was dropped off a cliff and hit the ground with a speed of 152 ft/s. What is the height of the cliff? (Use 32 ft/s for the acceleration due to gravity.) Step 1 We know that s(t) = 1 at + vot...
-
The 150 m long beam is submitted to a distributed load w(x) = (0.05 x 2) + 10 N/m. 50 50 w(x) 100 150 What is the moment about the point O in kN.m created by the distributed load? O-25.7 kN.m O-249...
-
Run an online search for two to three research firms that specialize in ethnography. Find sample ethnographic reports at the sites. Do you have confidence that these businesses produce credible...
-
By referring to Figure 13.18, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 oC: (a) KClO3, (b) Pb(NO3)2, (c) Ce2(SO4)3.
-
Which molecule would you expect to be more soluble in water: CCl 4 or CH 2 Cl 2 ?
-
Show which of the nitrogen atoms in purine are basic, and which one is not basic. For the non-basic nitrogen, explain why its nonbonding electrons are not easily available to become protonated.
-
The proton NMR spectrum of 2-pyridone gives the chemical shifts shown. (a) Is 2-pyridone aromatic? (b) Use resonance forms to explain your answer to (a). Also explain why the protons at (7.31and...
-
Explain why each compound is aromatic, antiaromatic, or nonaromatic. (a) (b) (c) (d) (e) (f) (g) (h) N: isoxazole 1.3-thiazole pyran pyrylium ion y-pyrone 1.2-dihydropyridine NH2 N:
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


