Explain the following phenomenon: You have a test tube with an aqueous solution of silver nitrate as
Question:
Explain the following phenomenon: You have a test tube with an aqueous solution of silver nitrate as shown in test tube 1 below. A few drops of aqueous sodium chromate solution was added with the end result shown in test tube 2. A few drops of aqueous sodium chloride solution was then added with the end result shown in test tube 3
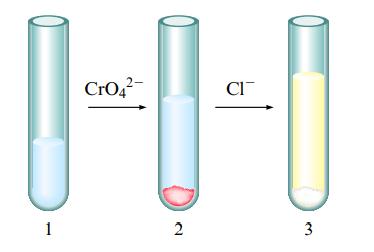
Use the Ksp values in the book to support your explanation, and include the balanced equations. Also, list the ions that are present in solution in each test tube.
Transcribed Image Text:
CrO4²- CIT |-|-·| 2 1 im 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Solution From above my explanation is that in the first tube test tube 1 the silver ...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
An aqueous solution of ammonium nitrite, NH4NO2, decomposes when heated to give off nitrogen, N2. NH4NO2(s) 2H2O(g) + N2(g) This reaction may be used to prepare pure nitrogen. How many grams of...
-
An aqueous solution of a molecular compound freezes at -0.086C. What is the molality of the solution?
-
An aqueous solution of antifreeze contains 6.067 M ethylene glycol (HOCH2CH2OH, FM 62.07) and has a density of 1.046 g/mL. (a) Find the mass of 1.000 L of this solution and the number of grams of...
-
Given the following data about XYZ Mutual Fund on Oct. 1: Assets: Liabilities: Cash = $40,000 Accrued fees and expenses = $5,000 1,000 Shares of Stock A: Closing Price $30 2,000 Shares of Stock B:...
-
Are refundings by corporations likely to occur steadily over time? If not, when are waves of refundings likely to occur?
-
Why are all returns on the CML perfectly negative correlated with the SDF?
-
Did the Squirrel Cage bartender act responsibly in the service of alcohol to Mr. Hadley? Did she act differently from bartenders in similar situations?
-
Traver-Dunlap Corporation has a 15% weighted average cost of capital (WACC). Its most recent sales were $980 million and its total net operating capital is $870 million. The following shows estimates...
-
The following is the membership funds for Marina cc at December 2019: Members Alistair Brian Clyde TOTALS Members interest 50% 30% 20% 100% Capital contributions: Cash N$ 10 000 N$ 8 000 N$ 6 000 N$...
-
A random variable X takes on three values, e.g., a, b, and c, with probabilities 0.55, 0.25, and 0.2. (a) What are the lengths of the binary Huffman codewords for X? What are the lengths of the...
-
Which of the following will affect the total amount of solute that can dissolve in a given amount of solvent? a. The solution is stirred. b. The solute is ground to fine particles before dissolving....
-
Consider a solution prepared by mixing the following: 50.0 mL of 0.100 M Na 3 PO 4 100.0 mL of 0.0500 M KOH 200.0 mL of 0.0750 M HCl 50.0 mL of 0.150 M NaCN Determine the volume of 0.100 M HNO 3 that...
-
In late 1991 two firms, Delta Airlines and the Trump Shuttle, provided air shuttle service between New York and Boston or Washington. The one-way price charged by both firms was $142 on weekdays and...
-
Choose a private label product that you have seen and discuss the possible reasons for why the particular retailer introduced this private label product and explain its features in detail.
-
Understanding your behaviors can help you become a better leader. As discussed in module 4 our beliefs & values can be summed up as our 'personality'. In this assignment you are to examine your own...
-
This week we learned about assessing competition. Watch the video the History of the Cola Wars and answer the following questions. Using the frameworks from the text and the online lesson, why is...
-
Prior to developing your training programs, you must analyze your organizational military needs, identify employee skills gaps based on performance, and have resources available to support training...
-
Describe specifically how your firm's culture lines up with the bullet points listed for that firm . For instance, if you believe your organization's strategy priority is creativity-driven , then...
-
Use the information given about the angle , 0 2 to find the exact value of (a) sin(2) (b) cos(2) (c) sin /2 (d) cos /2 sec = 2, csc < 0
-
At Glass Company, materials are added at the beginning of the process and conversion costs are added uniformly. Work in process, beginning: Number of units Transferred - in costs Direct materials...
-
In Chapter 10, we will see that an acetylide ion (formed by treatment of acetylene with a strong base) can serve as a nucleophile in an S N 2 reaction: This reaction provides a useful method for...
-
Predict the product(s) obtained when each of the following compounds is treated with chloromethane and aluminum trichloride. Some of the compounds might be unreactive. For those that are reactive,...
-
Predict the major product obtained when each of the following compounds is treated with bromine in the presence of iron tribromide. (a) Bromobenzene (b) Nitrobenzene (c) ortho-Xylene (d)...
-
[ The following information applies to the questions displayed below ] Nauticat has two classes of stock authorized: $ 1 0 par preferred, and $ 1 par value common. As of the beginning of 2 0 2 1 , 1...
-
Selling is not the most important part of marketing. Explain why not
-
When direct materials are issued from the storeroom, are any entries made in the subsidiary records? Question 2 options: Increase raw material item record Decrease raw material item record No entry...

Study smarter with the SolutionInn App


