For the following chemical reactions, determine the precipitate produced when the two reactants listed below are mixed
Question:
For the following chemical reactions, determine the precipitate produced when the two reactants listed below are mixed together. Indicate “none” if no precipitate will form.
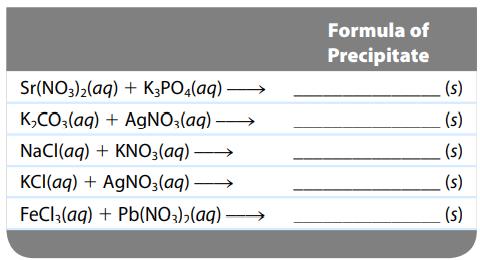
Transcribed Image Text:
Sr(NO3)₂(aq) + K3PO4(aq) →→→→ K₂CO3(aq) + AgNO3(aq) →→→→→→→ NaCl(aq) + KNO3(aq) KCl(aq) + AgNO3(aq) →→→→→→→ FeCl3(aq) + Pb(NO3)₂(aq) →→→→→→→→ Formula of Precipitate (s) (s) (s) (s) (s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
Formula of Precipitate SrNO32aq K3PO4aq Sr3PO42s KCO3aq A...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
The following chemical reactions take place in a liquid-phase batch reactor of constant volume V. A 2B r1 [mol A consumed/ (Ls)] = 0.100CA B C r2 [mol C generated/(Ls) = 0.200C2B where the...
-
The following chemical reactions take place in a closed system 2A + B ( C, A + D ( C. At equilibrium. They can be characterized by Where the nomenclature c i represents the concentration of...
-
Which of the following chemical reactions could be used to distinguish between a polyunsaturated vegetable oil and a petroleum oil containing a mixture of saturated and unsaturated hydrocarbons?...
-
1. Jowel, the financial manager for Berjayasama Bhd, wishes to evaluate three potential investments: Investment A, Investments B and Investment C. Table 1 shows the expected returns. You have been...
-
Bud received 200 shares of Georgia Corporation stock from his uncle as a gift on July 20, 2014, when the stock had a $45,000 FMV. His uncle paid $30,000 for the stock on April 12, 2000. The taxable...
-
Determine the median and the first and third quartiles in the following data. 6.02 7.30 11.86 7.99 12.71 5.24 6.67 10.39 7.59 12.22 8.03 13.07 8.35 13.59 8.81 13.89 9.45 15.42 10.37 9.61
-
Direct labour-rate variance is the difference between the standard direct wages specified for the activity achieved as the actual direct wages paid.
-
Fifteen years ago the Acme Manufacturing Company bought a propane-powered forklift truck for $4800. The company depreciated the forklift using straight line depreciation , a 12-year life, and zero...
-
192 193 194 On October 1, 2020 Devons Compnay declared a $2 per share cash dividend on common stock. Number of shares of $1 par common stock issued and outstanding 25,000 Number of shares of $100 par...
-
Effective financial statement analysis requires an understanding of a firms economic characteristics. The relations among various financial statement items provide evidence of many of these economic...
-
A 500.0-mL sample of 0.200 M sodium phosphate is mixed with 400.0 mL of 0.289 M barium chloride. What is the mass of the solid produced?
-
A solution is prepared by dissolving 0.6706 g oxalic acid (H 2 C 2 O4) in enough water to make 100.0 mL of solution. A 10.00-mL aliquot (portion) of this solution is then diluted to a final volume of...
-
Compute the elevations of points A and B in Problem 27.20.
-
For the past 30 years, the average satisfaction rating for a sushi restaurant has been 3.9 out of 5. If the rating for a sample of 256 people is 4.1 with a standard deviation of 0.5, the critical...
-
Hash collisions occur when more than one item is mapped to the same element in Hash Table's array. What is one way that a Hash Table can handle collisions?
-
Scatterplot. In Exercises 5-8, use the sample data to construct a scatterplot. Use the first variable for the x-axis. Based on the scatterplot, what do you conclude about a linear correlation? Pulse...
-
Given two fair six sided dice and a standard deck of 52 playing cards, calculate the probability of a rolling a sum of 7 or 11 and drawing three cards in which at least one is a face card.
-
z Scores. In Exercises 5-8, express all z scores with two decimal places. 5. Diastolic Blood Pressure of Females For the diastolic blood pressure measurements of females listed in Data Set 1 "Body...
-
1. If poverty is defined in absolute terms, will the list of necessities still vary from society to society? 2. If we use a relative definition of poverty, what difficulties would we encounter in...
-
Archangel Corporation prepared the following variance report. Instructions Fill in the appropriate amounts or letters for the question marks in the report. ARCHANGEL CORPORATION Variance...
-
The collision frequency z of a molecule of mass m in a gas at a pressure pis z = 4(J (kTlnm) 1/2p/kT, where o is the collision cross-section. Find an expression for the collision-limited lifetime of...
-
The rotational constant for CO is 1.9314 cm-1 and 1.6116 cm-1 in the ground and first excited vibrational states, respectively. By how much does the intern clear distance change as a result of this...
-
Rotational absorption lines from 1H35CI gas were found at the following wave numbers (R.1, Hausler and R.A. Oetjen, f. Chem. Phys. 21, 1340 (1953)): 83.32, 104.13, 124.73, 145.37, 165.89, 186.23,...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...
-
5. The current spot exchange rate is 0.95/$ and the three-month forward rate is 0.91/$. Based on your analysis of the exchange rate, you are pretty confident that the spot exchange rate will be...

Study smarter with the SolutionInn App


