The following chemical reactions take place in a closed system 2A + B ( C, A +
Question:
The following chemical reactions take place in a closed system 2A + B ( C, A + D ( C. At equilibrium. They can be characterized by
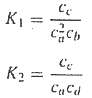
Where the nomenclature ci represents the concentration of constituent i. If x1 and x2 are the number of moles of C that are produced duo to the first and second reactions, respectively, use an approach similar to that of Prob. 8.5 to reformulate the equilibrium relationship in terms of the initial concentrations of the constituents. Then, use the Newton-Raphson method to solve the pair of simultaneous nonlinear equations for x1 and x2?if K1 = 4 x 10-4, K2 = 3.7 x 10-2, ca,0 = 50, cb,0 = 20, cc,0 = 5, cd,0 = 10. Use a graphical approach to develop your initial guesses.
Step by Step Answer:

Numerical Methods For Engineers
ISBN: 9780071244299
5th Edition
Authors: Steven C. Chapra, Raymond P. Canale





