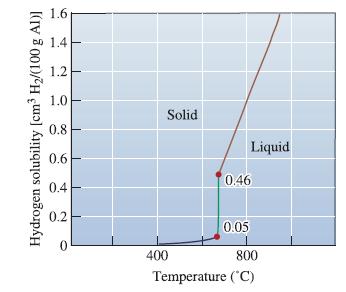
From Figure 9-14, find the solubility of hydrogen in liquid aluminum just before solidification begins when the
Question:
From Figure 9-14, find the solubility of hydrogen in liquid aluminum just before solidification begins when the partial pressure of hydrogen is 1 atm. Determine the solubility of hydrogen (in cm3/100 g Al) at the same temperature if the partial pressure were reduced to 0.01 atm.
Transcribed Image Text:
Hydrogen solubility [cm³ H₂/(100 g Al)] 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.2 Solid 400 Liquid 0.46 0.05 800 Temperature (°C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
The solubility of hydrogen in cm3100 g Al 3365 00677 x 1 atm 00117 ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
The solubility of hydrogen in liquid aluminum at 715C is found to be 1 cm 3 / (100 g Al). If all of this hydrogen precipitated as gas bubbles during solidification and remained trapped in the...
-
Repeat Prob. 965E if the compression ratio were reduced to 12. Data From Q#65: An air-standard dual cycle has a compression ratio of 20 and a cutoff ratio of 1.3. The pressure ratio during the...
-
The solubility of hydrogen gas in steel in terms of its mass fraction is given as WH, = 2.09 X 104 exp(-3950/T)P p05 where PH2 is the partial pressure of hydrogen in bars and T is the temperature in...
-
Suppose that a firm is producing in the short run with output given by: Q = 200.5L 2.5L 2 , The firm hires labor at a wage of $25 per hour and sells the good in a competitive market at P = $50 per...
-
Find the degree of intra-industry trade if exports and imports are, respectively (a) 1,000 and 1,000 (b) 1,000 and 750 (c) 1,000 and 500 (d) 1,000 and 25 (e) 1,000 and 0
-
1. Identify the psychological phenomena in the minicase. Prioritize the phenomena from most important to least important. Begin your answer by defining the phenomena, and then describing their role...
-
Which of these statements is true regarding product and period costs? 1. Factory maintenance is a product cost and sales commission is a period cost. 2. Sales commission is a product cost and...
-
Multiple Choice Questions 1. Which of the following combinations correctly describes the relationship between foreign currency transactions, exchange rate changes, and foreign exchange gains and...
-
Lawn and Trees Company purchased a new aerial tree trimmer at $91,000 and paid $1,800 for its shipping and $1,500 for its assembly and initial training. If the salvage value at the end of 10 years is...
-
The Fashion Rack has a monthly accounting period. All transactions are recorded in a general journal. Postings are made from the general journal to the accounts receivable ledger, accounts payable...
-
Explain the green sand molding process.
-
Why is it that castings made from pressure die casting are likely to be stronger than those made using the sand casting process?
-
Tam wanted to open a Vietnamese take-out restaurant. He has a degree in Business Administration from a reputable west coast university and worked most of his life in his parents Vietnamese (eat-in)...
-
Carla Vista Corp. sponsors a defined benefit pension plan for its employees. On January 1, 2025, the following balances relate to this plan Plan assets $489,900 Projected benefit obligation 616,700...
-
Question 2 of 8 Shirts were purchased for $12.50 each and were marked up by $18.75. During Christmas, they were discounted by $6.85 per shirt. a. What was the rate of markdown? % Round to two decimal...
-
The cost versus quality decision is one that only few companies get right. What is the cost of quality? It is very high for some companies such as Ford and Bridgestone/Firestone, whose reputations...
-
Find the absolute maximum and absolute minimum values of the function f(x) (x-2)(x-5)+7 = on each of the indicated intervals. Enter 'NONE' for any absolute extrema that does not exist. (A) Interval =...
-
4. Roll one 10-sided die 12 times. The probability of getting exactly 4 eights in those 12 rolls is given by (a) 10 9 4 10 10 (b) HA 9 -HAA (c) 1 (d) 9 (c) 10 9 () 10
-
Think of all the tasks that you perform when you purchase a car. Include any research, decisions, or financial issues that relate to the purchase. Draw a Gantt chart that shows all the tasks and the...
-
Briefly describe the following types of group life insurance plans: a. Group term life insurance b. Group accidental death and dismemberment insurance (AD&D) c. Group universal life insurance d....
-
Give three examples of engineered products that are designed to work well over a million times.
-
Select a type of product that would have versions in all four quadrants in the style-versus-technology design chart (see Focus On Innovation). Show the four versions of the product, and clearly...
-
Imagine you are tasked with designing a coffee maker that would be marketable to cafs around the globe. Conduct research on coffee makers to determine a set of global, social, environmental, and...
-
Ray Company provided the following excerpts from its Production Department's flexible budget performance report. Required: Complete the Production Department's Flexible Budget Performance Report....
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...

Study smarter with the SolutionInn App


